149-57-5
- Product Name:2-Ethylhexanoic acid
- Molecular Formula:C8H16O2
- Purity:99%
- Molecular Weight:144.214
Quality Factory Supply 2-Ethylhexanoic acid factory price(149-57-5) 149-57-5
- Molecular Formula:C8H16O2
- Molecular Weight:144.214
- Appearance/Colour:colourless liquid
- Vapor Pressure:<0.01 mm Hg ( 20 °C)
- Melting Point:-59 °C
- Refractive Index:n20/D 1.425(lit.)
- Boiling Point:228 °C at 760 mmHg
- PKA:pK1:4.895 (25°C)
- Flash Point:116.6 °C
- PSA:37.30000
- Density:0.926 g/cm3
- LogP:2.28740
2-Ethylhexanoic acid(Cas 149-57-5) Usage
|
General Description |
2-Ethylhexanoic acid is a colorless to light yellow liquid with a mild odor. 2-Ethylhexanoic acid is the organic compound which is a carboxylic acid that is widely used to prepare lipophilic metal derivatives that are soluble in nonpolar organic solvents. |
|
Uses |
2-Ethylhexanoic acid is widely used in esters for PVB film plasticizers and synthetic lubricants, in production of metal soaps for paint driers, in automotive coolants and PVC stabilizers. Other application areas include wood preservatives, catalyst for polyurethane and in pharmaceuticals. |
|
Fire Hazard |
2-Ethylhexanoic acid is combustible. |
|
Flammability and Explosibility |
Notclassified |
|
Safety Profile |
Moderately toxic by ingestion and skin contact. An experimental teratogen. A skin and severe eye irritant. Combustible when exposed to heat or flame. When heated to decomposition, it emits acrid and irritating fumes. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C8H16O2/c1-3-5-6-7(4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10)/p-1/t7-/m1/s1
149-57-5 Relevant articles
Preparative microdroplet synthesis of carboxylic acids from aerobic oxidation of aldehydes
Yan, Xin,Lai, Yin-Hung,Zare, Richard N.
, p. 5207 - 5211 (2018)
Single liquid-phase and liquid-liquid ph...
The Regiospecific Palladium Catalysed Hydrocarboxylation of Alkenes under Mild Conditions
Alper, Howard,Woell, James B.,Despeyroux, Bertrand,Smith, David J. H.
, p. 1270 - 1271 (1983)
Alkenes react with carbon monoxide, wate...
Efficient CuCl-catalyzed selective and direct oxidation of β- And γ-substituted aliphatic primary alcohols to carboxylic acids
Mannam, Sreedevi,Sekar, Govindasamy
, p. 2822 - 2829 (2010)
A new procedure for the selective and di...
Physicochemical properties of 2-ethylhexanoic acid N′,N′- dialkylhydrazides
Radushev,Batueva,Gusev
, p. 1196 - 1200 (2006)
The following characteristics of 2-ethyl...
Reinvestigation of the Organocatalyzed Aerobic Oxidation of Aldehydes to Acids
Vanoye, Laurent,Abdelaal, Mohamed,Grundhauser, Kacy,Guicheret, Boris,Fongarland, Pascal,De Bellefon, Claude,Favre-Réguillon, Alain
, p. 10134 - 10138 (2019)
The organocatalyzed aerobic oxidation of...
Mechanistic Insights into the Aerobic Oxidation of Aldehydes: Evidence of Multiple Reaction Pathways during the Liquid Phase Oxidation of 2-Ethylhexanal
Vanoye, Laurent,Favre-Réguillon, Alain
, p. 335 - 346 (2022/02/10)
The liquid-phase aldehyde oxidation by m...
N-Heterocyclic Carbene/Carboxylic Acid Co-Catalysis Enables Oxidative Esterification of Demanding Aldehydes/Enals, at Low Catalyst Loading
Berkessel, Albrecht,Biswas, Animesh,Harnying, Wacharee,Sudkaow, Panyapon
supporting information, p. 19631 - 19636 (2021/08/09)
We report the discovery that simple carb...
Method for producing aliphatic carboxylic acid compound and pyridine compound adduct of aliphatic ketone compound
-
Paragraph 0172; 0175-0176; 0182; 0185-0186; 0192; 0195-0196, (2020/05/02)
Provided are: a method for producing an ...
Preparation method of bimetallic catalyst oxidation aldehyde synthetic carboxylic acid (by machine translation)
-
Paragraph 0050-0051, (2020/05/30)
The method is, in a reaction solvent: un...
149-57-5 Upstream products
-
79-21-0
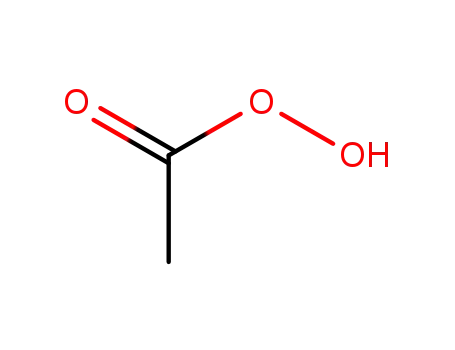
peracetic acid
-
123-05-7
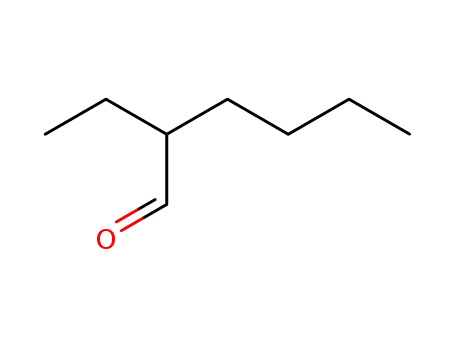
d,l-2-ethylhexanal
-
104-76-7
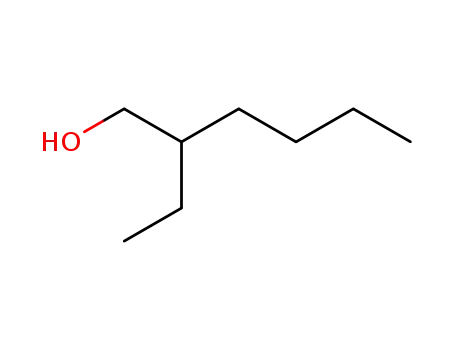
2-Ethylhexyl alcohol
-
645-62-5
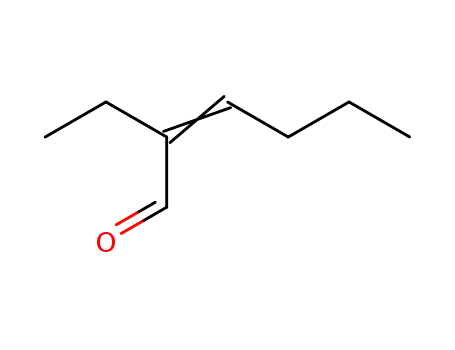
2-ethylhexenal
149-57-5 Downstream products
-
5451-25-2
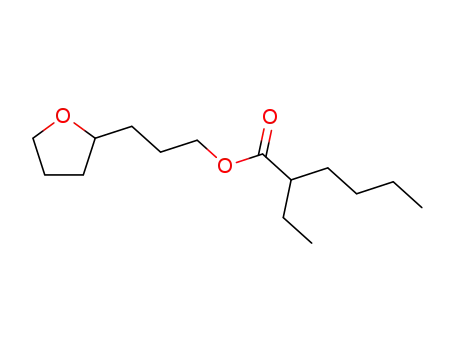
2-ethyl-hexanoic acid-(3-tetrahydro[2]furyl-propyl ester)
-
6807-05-2
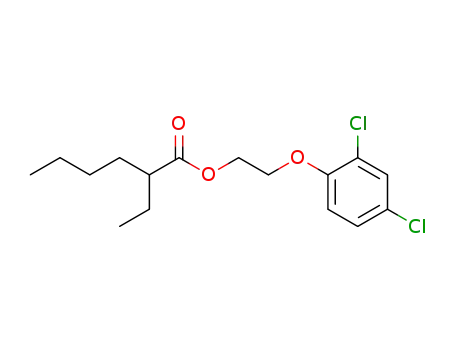
1-(2-ethyl-hexanoyloxy)-2-(2,4-dichloro-phenoxy)-ethane
-
36765-89-6
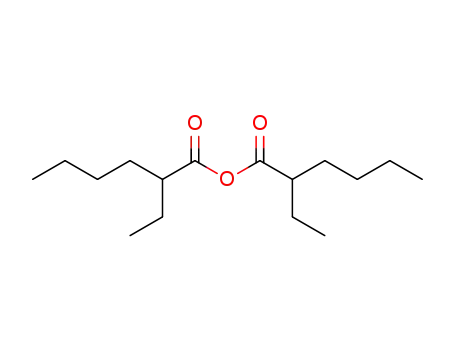
2-ethylhexanoic acid anhydride
-
760-67-8
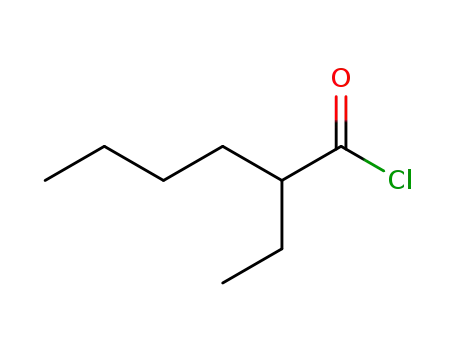
2-ethylhexanoic acid chloride