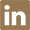463-40-1
- Product Name:Linolenic acid
- Molecular Formula:C18H30O2
- Purity:99%
- Molecular Weight:278.435
Reputable factory supply Linolenic acid 463-40-1 in bulk at low price
- Molecular Formula:C18H30O2
- Molecular Weight:278.435
- Appearance/Colour:clear light yellow to yellow liquid
- Vapor Pressure:4.24E-09mmHg at 25°C
- Melting Point:-11 °C(lit.)
- Refractive Index:1.479 - 1.481
- Boiling Point:443.376 °C at 760 mmHg
- PKA:4.78±0.10(Predicted)
- Flash Point:275.733 °C
- PSA:37.30000
- Density:0.925 g/cm3
- LogP:5.66050
Linolenic acid(Cas 463-40-1) Usage
|
Overview |
The alpha-linolenic acid (ALA 18:3w) an omega-3 (n-3) fatty acid, is an essential fatty acid (EFA) that cannot be synthesized by the body and therefore must be supplied by dietary sources. ALA is abundant in certain plant foods including walnuts, rapeseed (canola), several legumes, flaxseed, and green leafy vegetables.[1] ALA is the precursor of three important longer-chain n-3 fatty acids, eicosapentaenoic acid (EPA 20:5w3), docosapentaenoic acid (DPAw3 22:5w3), and docosahexaenoic acid (DHA 22:6w3), which have vital roles in brain development and function, cardiovascular health, and inflammatory response.[2-5] Omega-3 fatty acids are incorporated into the membrane lipid bilayer in virtually all body cells and affect membrane composition, eicosanoid biosynthesis, cell signaling cascades, and gene expression[6]. |
|
Absorption and metabolism of ALA |
As a rule, dietary fats are absorbed very efficiently from the digestive tract, and ALA is no exception. Burdge 16 recently reported that absorption levels of ALA are 96% or more. There are several possible metabolic fates for ALA that enters the bloodstream. The body can store the fatty acids in adipose tissue, use them for acetyl-CoA or energy production through b-oxidation, synthesize other non-essential saturated or monounsaturated fatty acids (MUFA), or convert them to longer-chain n-3 polyunsaturated fatty acids (PUFA) in the liver. The activity of the desaturation/elongation pathway is of unique importance as it is responsible for the synthesis of EPA and DHA. What is presently understood concerning conversion of ALA to EPA, DPAw3, and DHA is that the first step in the pathway, addition of a fourth double bond by D 6 desaturase, is considered to be the rate-limiting step. This is followed by elongation (addition of two carbon atoms) and an additional desaturation by the enzyme D 5 desaturase, with the product being 20:5n-3 or EPA. Several possibilities have been suggested for the precise pathway for production of DHA (22:6n-3) from EPA in humans. It was assumed that the conversion of DPAw3 to DHA would be carried out by a D4 desaturase but, thus far, little or no D4 desaturase has been found. Sprecher[8] has provided evidence for elongation of 22:5w3 to 24:5w3,which is then desaturated by the rate limiting D 6 desaturase to yield a 24:6w3. Two carbons are then cleaved in the peroxisomes to yield DHA that is then exported to the reticuloendothelial system. Thus, the insertion of the last double bond in DHA production in human metabolism may be rather indirect and somewhat inefficient. There are some doubts as to the validity of the Sprecher shunt, but it provides a possible explanation for the small proportions of DHA produced and the build up of DPAw3, despite ample ALA available in foods and in body tissues. Alpha-linolenic acid is partially converted to EPA in humans (8–20%), while conversion rates of ALA to DHA are estimated at 0.5–9%[9, 10]. The sex difference in metabolism is well known. Studies in women of reproductive age showed a substantially greater (2.5-fold) rate of conversion of ALA to EPA than that measured in healthy men. Thus, the ability to produce long-chain metabolites is gender dependent. It appears that women have a lower partitioning of ALA to b-oxidation, leaving more of it available for conversion to EPA[7, 11]. Other possible explanations include a direct effect of estrogen on conversion rates[7, 12]. Gender differences have also been observed in the conversion rates of ALA to DHA. In males it is estimated that only 0.5–4% of ALA is converted to DHA while in females the rates are thought to be as high as 9%[11, 12]. It is hypothesized that demands for DHA by the fetus during pregnancy may stimulate female physiology to more readily synthesize this fatty acid. |
|
Biological effects of ALA |
Cardio-protective effect There are several studies that strongly indicate that ALA may indeed be important in maintaining heart health. Because ALA is a precursor of the longer chained n-3 fatty acids, it contributes to the body pools of EPA and DHA that have been associated with improved vascular tone, heart rate, serum lipid levels, platelet function, inflammatory responses, arrhythmia, growth rates of atherosclerotic plaques, and blood pressure[13-15]. Most of these beneficial effects have also been associated specifically with ALA consumption. Interest in the role of ALA in the prevention of heart disease arose following the publication of the Lyon Heart Study carried out in France[16] This study provided the first evidence that ALA consumption may play an important role in reduction of heart disease. In this secondary prevention trial, which included 608 patients with known CHD, participants in the intervention group were encouraged to follow the Mediterranean diet and were supplied with canola margarine rich in ALA. At 27-month follow-up, a 73% reduction in cardiovascular events was observed along with a 70% decrease in overall mortality. ALA intake in the intervention group was three times higher than that in the control subjects. Although it appears that ALA may have contributed to the improvement in cardiac health, there were numerous independent variables in the study, which makes it impossible to isolate the effects of ALA per se. Mozaffarian[13] reviewed the findings from observational studies that evaluated the relationship between ALA intake and risk of CHD or mortality. In most studies, particularly those in the American population, an inverse association between ALA consumption and incidence of CHD was found. For example, the Cardiovascular Health Study[17] which included a prospective cohort of close to 6000 older men and women, found that each one standard deviation increase in plasma phospholipid ALA levels (a biomarker of ALA intake) was associated with a 52% decrease in risk for fatal CHD. In The Nurses Health Study[18, 19] over 75,000 women were followed using dietary intake records to assess ALA consumption. ALA intake was associated with a significantly lower risk of sudden cardiac death. These results help support the hypothesis that ALA may have antiarrhythmic properties. Inflammation In a previous study, men suffering from dyslipidemia were provided a high-ALA diet (canola oil 15 ml/day) or a safflower oil control diet[20]. After 3 months, in individuals consuming the canola oil diet, systemic inflammation was significantly reduced, as measured by C-reactive protein, serum amyloid A, and interleukin-6. Additional evidence that ALA consumption affects biomarkers of inflammation and endothelial activation was observed in a crosssectional study of 727 women in the Nurses Health Study[21]. An inverse relationship was observed between ALA intake and plasma concentrations of C-reactive protein, IL-6, E-selectin, soluble intercellular cell adhesion molecule 1, and soluble vascular cell adhesion molecule 1. This provides support for the role of ALA as an antiinflammatory agent that also lowers endothelial activation. Ferrucci et al[22] also reported that higher levels of ALA in the blood were associated with lower levels of inflammatory biomarkers. His study included 1123 individuals, aged 20–98 years. Fatty acids in fasting plasma were analyzed in relation to numerous inflammatory markers. Decreased levels of C-reactive protein and IL-1ra levels were associated with higher plasma ALA levels. Zhao et al[23] have shown that dietary ALA elicits antiinflammatory effects by inhibiting IL-6, IL-1beta, and TNF-alpha production in peripheral blood mononuclear cells cultured from hypercholesterolemic individuals exposed to a diet high in ALA (6.5% of energy). Thus, consumption of ALA may provide protection against heart disease and other inflammatory diseases by reduction of inflammatory cytokines. Other health benefits Attention deficit hyperactivity disorder: A recent study by Joshi et al.[24] reported a significant improvement in the symptoms of ADHD in children that had received flax oil and Vitamin C supplements. Neuroprotection: Lauritzen et al.[25] reported that ALA prevented neuronal death in an animal model of transient global ischemia; it also protected animals treated with kainate against seizures and hippocampal lesions. An additional study reported neuroprotective effects in rat models of spinal cord ischemia leading to paraplegia[26]. ALA treatment preserved neurological function and animals sustained only mild-to-moderate injury. This suggests that ALA can induce protection against ischemia in spinal injury, preventing necrosis and apoptosis of motor neurons. Autoimmune diseases: Work in animal models by Reiffen et al[27] suggests that the addition of flaxseed oil (70% ALA) to the diet may attenuate the severity of systemic lupus erythematosus (SLE or lupus). SLE mice fed flaxseed exhibited lower titers of antibodies to DNA and to cardiolipin and less severe kidney damage than mice fed other diets, including fish oil. |
|
Adverse reactions |
However, concern has arisen from one metaanalysis that reported an increased incidence of prostate cancer risk in men with high intake or high blood levels of ALA (combined relative risk 1.70; 95% CI 1.12–2.58). The data presented was heterogeneous and the authors themselves stated it is uncertain if the results present a genuine effect. The results have yet to be confirmed. More recent reviews did not confirm or refute this possible deleterious effect of ALA. |
|
Definition |
A liquid unsaturated carboxylic acid that occurs in LINSEED OIL and other plant oils. It contains three double bonds. |
|
General Description |
Clear colorless liquid. |
|
Air & Water Reactions |
Oxidizes in air to form peroxides, which spontaneously ignite. Insoluble in water. Sensitive to heat and moisture. |
|
Reactivity Profile |
Linolenic acid is incompatible with bases, oxidizing agents and reducing agents. Also incompatible with peroxides, oxygen and water. |
|
Fire Hazard |
Linolenic acid is probably combustible. |
|
Biochem/physiol Actions |
An ω-3 fatty acid that serves as a precursor to eicosapentaenoic acid (EPA) but not docosahexaenoic acid. Conversion is greater in women than men, and conversely, β-oxidation metabolism is greater in men than women. |
|
Carcinogenicity |
Narisawa et al. found that diets high in perilla oil (with high a-linolenic acid but also high levels of linoleic acid) lowered the risk of colon cancer in a rat model initiated with the carcinogen N-methyl-Nnitrosourea. Okuno found a suppressive effect on diethylnitrosamine- induced heptaocarcinogenesis in male Fischer 344 rats treated with either safflower (high in linoleic acid) or perilla oil (high in both linoleic and α-linolenic acids). Kitano et al. found that 10% α-linolenic acid did not promote urninary bladder cancer initiated in rats with N-butyl-N-(4-hydrocybutyl)nitrosamine. Mice fed a-linolenic acid at 5–10% of the diet had increased mammary gland ductular cell proliferation. |
InChI:InChI=1/C18H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h3-4,6-7,9-10H,2,5,8,11-17H2,1H3,(H,19,20)/b4-3-,7-6-,10-9-
463-40-1 Relevant articles
Alteration of Chain Length Selectivity of Candida antarctica Lipase A by Semi-Rational Design for the Enrichment of Erucic and Gondoic Fatty Acids
Zorn, Katja,Oroz-Guinea, Isabel,Brundiek, Henrike,D?rr, Mark,Bornscheuer, Uwe T.
, p. 4115 - 4131 (2018/10/02)
Biotechnological strategies using renewa...
Linolenic acid-modified PEG-PCL micelles for curcumin delivery
Song, Zhimei,Zhu, Wenxia,Liu, Na,Yang, Fengying,Feng, Runliang
, p. 312 - 321 (2014/06/24)
In this study, a novel linolenic acid-mo...
A nine carbon homologating system for skip-conjugated polyenes
Mustafa, Hussein H.,Baird, Mark S.,Al Dulayymi, Juma'A R.,Tverezovskiy, Viacheslav V.
, p. 34 - 42 (2014/07/08)
Ozonolysis of Z,Z,Z-cylonona-1,4,7-trien...
Three new sucrose fatty acid esters from Equisetum hiemale L.
Cheng, Jin-Tang,Li, Yan,He, Juan,Li, Xing-Yao,Wu, Xing-De,Shao, Li-Dong,Dong, Liao-Bin,Deng, Xu,Gao, Xiu,Peng, Li-Yan,Cheng, Xiao,Zhao, Qin-Shi
experimental part, p. 1158 - 1163 (2012/09/22)
Three new monosubstituted sucrose fatty ...
463-40-1 Process route
-
linseed oil
-
linseed oil

-
-
9-cis-13-trans-15-cis-octadecatrienoic acid

-
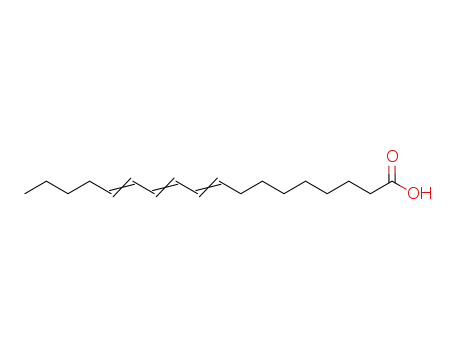
- 13296-76-9
9,11,13-octadecatrienoic acid

-
- 104096-79-9
10,12,14-C18:3

-
- 25574-96-3
10E,12Z,14E-C18:3

-
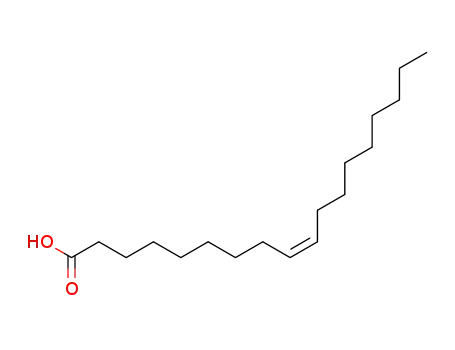
- 112-80-1,2027-47-6
cis-Octadecenoic acid

-
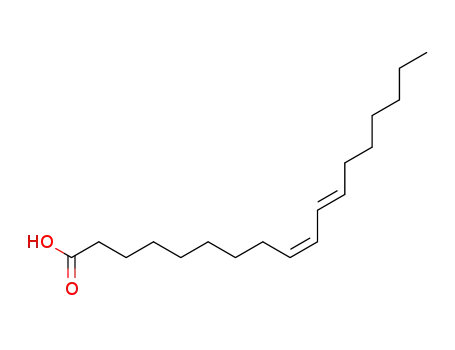
- 544-70-7,544-71-8,872-23-1,1839-11-8,2540-56-9
cis-9,trans-11-octadecadienoic acid

-
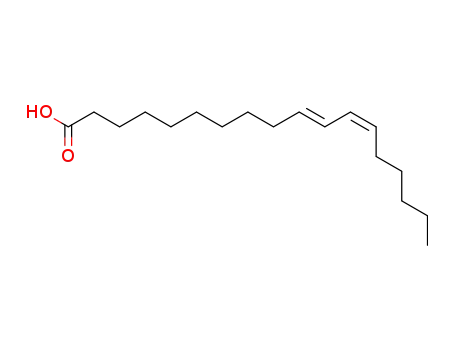
- 1072-36-2,2420-44-2,2420-56-6,7307-45-1,22880-03-1
trans-10,cis-12-octadecadienoic acid

-
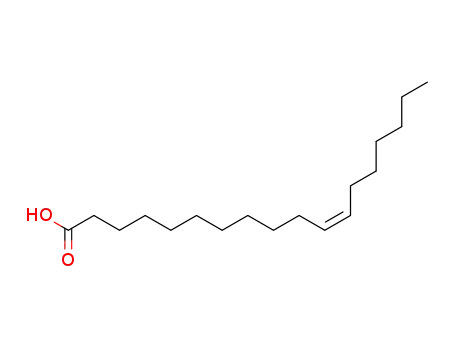
- 506-17-2
cis-vaccenic acid

-
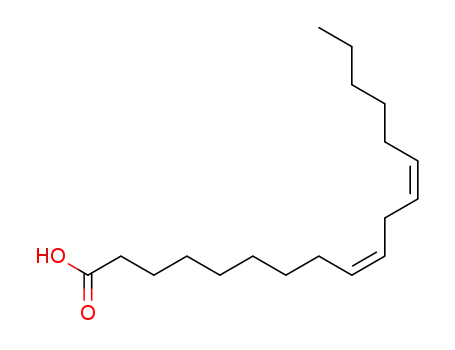
- 60-33-3,116745-32-5,26085-09-6,64080-88-2,7049-66-3,949900-18-9,60476-23-5,6144-28-1,67922-65-0,89718-18-3
linoleic acid

-
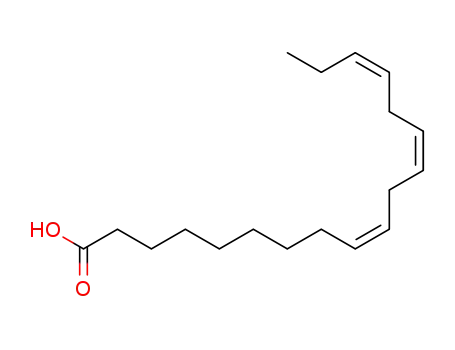
- 463-40-1,1955-33-5,21661-10-9,21661-09-6,21661-13-2,21661-11-0
(9Z,12Z,15Z)-octadeca-9-12,15-trienoic acid

-
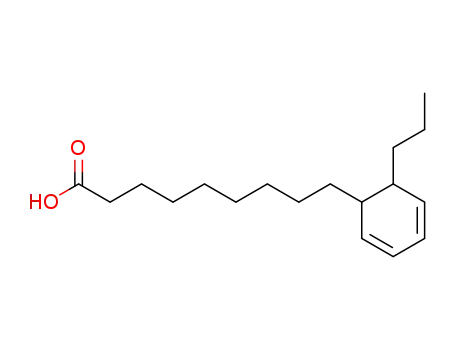
- 25491-26-3
9-(6-propyl-cyclohexa-2,4-dienyl)-nonanoic acid

-
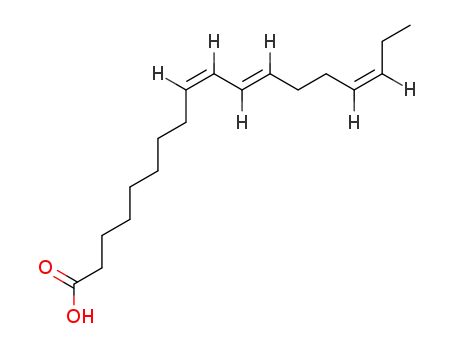
- 15909-18-9
rumelenic acid

-
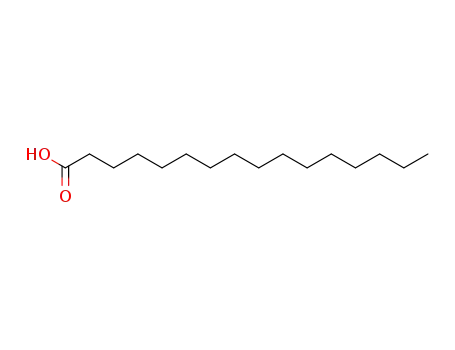
- 57-10-3
1-hexadecylcarboxylic acid

-
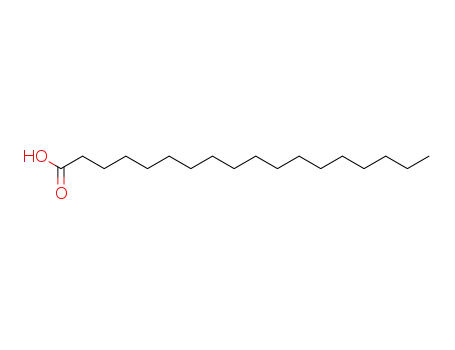
- 57-11-4
stearic acid
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In propylene glycol; at 160 ℃; for 2h;
|
-
borage oil
-
borage oil

-
- 657403-47-9
6Z,8E,12Z-C18:3

-
-
7E,9Z,11E-C18:3

-
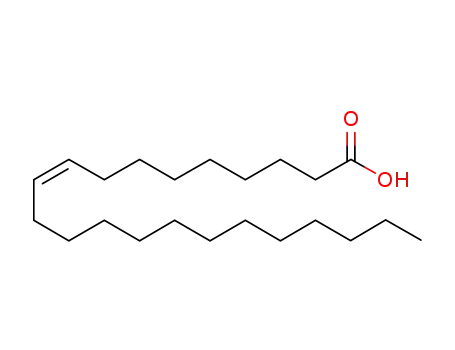
- 25692-11-9,14134-53-3
cis-Δ9-docosenoic acid

-

- 112-80-1,2027-47-6
cis-Octadecenoic acid

-

- 544-70-7,544-71-8,872-23-1,1839-11-8,2540-56-9
cis-9,trans-11-octadecadienoic acid

-
- 1072-36-2,2420-44-2,2420-56-6,7307-45-1,22880-03-1
trans-10,cis-12-octadecadienoic acid

-

- 506-17-2
cis-vaccenic acid

-

- 60-33-3,116745-32-5,26085-09-6,64080-88-2,7049-66-3,949900-18-9,60476-23-5,6144-28-1,67922-65-0,89718-18-3
linoleic acid

-
- 29204-02-2,506-31-0
gadoleic acid

-

- 463-40-1,1955-33-5,21661-10-9,21661-09-6,21661-13-2,21661-11-0
(9Z,12Z,15Z)-octadeca-9-12,15-trienoic acid

-
- 57-10-3
1-hexadecylcarboxylic acid

-
- 57-11-4
stearic acid

-
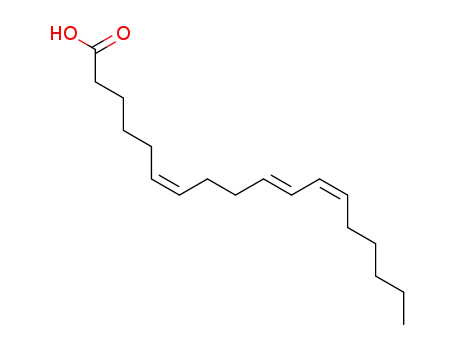
- 109241-60-3
(6Z,10E,12Z)-octadeca-6,10,12-trienoic acid
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In propylene glycol; at 160 ℃; for 2h;
|
463-40-1 Upstream products
-
4167-08-2
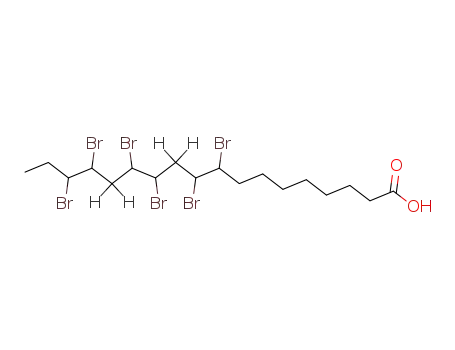
9,10,12,13,15,16-hexabromo-octadecanoic acid
-
64-17-5
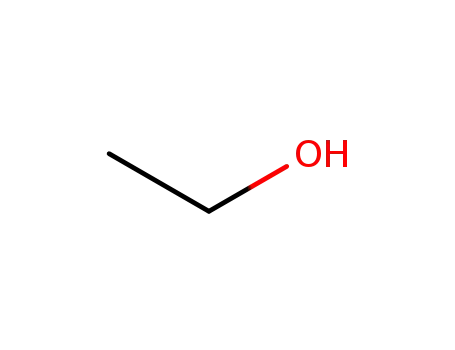
ethanol
-
60-33-3

linoleic acid
-
145937-22-0
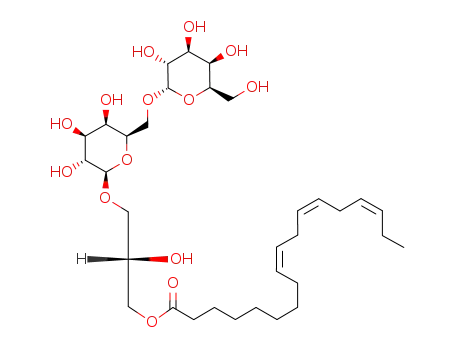
(2S)-3-O-[α-D-galactopyranosyl-(1->6)-β-D-galactopyranosyl]-1-O-[(9Z,12Z,15Z)-octadeca-9,12,15-trienoyl]-sn-glycerol
463-40-1 Downstream products
-
112-62-9
octadec-9-enoic acid methyl ester
-
29972-79-0
methyl 11-dodecenoate
-
112-39-0
hexadecanoic acid methyl ester
-
112-61-8
Methyl stearate