Reputable factory supply Shikonin 517-89-5 in bulk at low price
- Molecular Formula:C16H16O5
- Molecular Weight:288.3
- Vapor Pressure:1.04E-13mmHg at 25°C
- Melting Point:147 °C
- Refractive Index:1.642
- Boiling Point:567.4 °C at 760 mmHg
- PKA:7.34±0.20(Predicted)
- Flash Point:311 °C
- PSA:94.83000
- Density:1.373 g/cm3
- LogP:2.12040
5,8-Dihydroxy-2-[(1R)-1-hydroxy-4-methyl-pent-3-enyl]naphthalene-1,4-dione(Cas 517-89-5) Usage
|
History |
Kuroda and Majima firstly identified acetyl shikonin from L. erythrorhizon in 1922 , followed by the discovery of other shikonin derivatives, including shikonin. The chemical structure of shikonin was not precisely identified till 1936 for its high physicochemical similarity with naphthazarin . There have been about 500 publications on shikonin up to now. Great attention has also been paid on the biosynthesis of shikonin and its derivatives, and an increasing number of shikonin derivatives have been designed and synthesized for exploring their antitumor effect. There are two types of derivatives: one is modifications of 1′-OH with parent nucleus naphthazarin remained and the other is modifications of both 1′-OH and parent nucleus naphthazarin, as shown in Fig. 3c, d . |
|
Pharmacology |
Shikonin possesses anti-inflammatory, antioxidant, antiviral, cardiovascular protective,and antitumor activities. Shikonin reduces inflammation by inhibiting the biosynthesis of leukotrienes and 5-hydroxyeicosatetraenoic acid and thus reduces synthesis of inflammation-related active molecules, which selectively block chemokine binding to CC chemokine receptor 1 . Shikonin shows free radical scavenging and antioxidant (especially toward superoxide anion and DPPH) activities. It significantly inhibits autoxidation caused by β-carotene and linoleic acid . Its anti-HCV effects have been reported recently with an EC50 at 25 ng/mL, which is lower than that of ribavirin (2.6 μg/mL) . Recent studies also reveal shikonin possesses cardiovascular protective effects. Shikonin inhibits the activity of TNF-α promoter, revealing its transcriptional antagonism to pro-inflammatory cytokine . Shikonin also shows antitumor potentials by inducing apoptosis and/or necrosis, inhibiting DNA topoisomerase activity and angiogenesis, and regulating proliferative signaling pathways (including MAPK, VEGF, and PTKs ). In addition, shikonin circumvents cancer drug resistance by induction of necroptotic cell death . |
|
Physical properties |
Appearance: purple lamellae or crystalline powder. Melting point: 147 °C. Specific optical rotation: +138°(benzene). Solubility: soluble in ethanol, vegetable oils, and other organic solvents. |
|
General Description |
This substance is a primary reference substance with assigned absolute purity (considering chromatographic purity, water, residual solvents, inorganic impurities). The exact value can be found on the certificate. Produced by PhytoLab GmbH & Co. KG |
InChI:InChI=1/C16H16O5/c1-8(2)3-4-10(17)9-7-13(20)14-11(18)5-6-12(19)15(14)16(9)21/h3,5-7,10,17-19H,4H2,1-2H3/t10-/m1/s1
517-89-5 Relevant articles
A novel and efficient total synthesis of shikonin
Wang, Rubing,Guo, Hui,Cui, Jiahua,Li, Shaoshun
, p. 3977 - 3980 (2012)
A novel and efficient synthesis of shiko...
High performance liquid chromatography resolution method of alkannin raceme and naphthazarin parent nucleus hydroxyl methylated carbonyl oxime derivative of alkannin raceme
-
Paragraph 0036-0045, (2021/04/17)
The invention discloses a high performan...
An improved and practical synthesis route to antiproliferative (±)-shikonin and its O-acyl derivatives
Ono, Mana,Abe, Shouki,Higai, Koji,Higashi, Shoko,Saito, Setsuo,Saito, Ryota
, p. 738 - 746 (2020/12/09)
Shikonin and its O-acyl derivatives are ...
An efficient multigram synthesis of alkannin and shikonin
Wang, Rubing,Zhou, Shanshan,Jiang, Hudagula,Zheng, Xiaogang,Zhou, Wen,Li, Shaoshun
supporting information; experimental part, p. 1373 - 1379 (2012/04/04)
The concise and efficient total synthese...
517-89-5 Process route
-
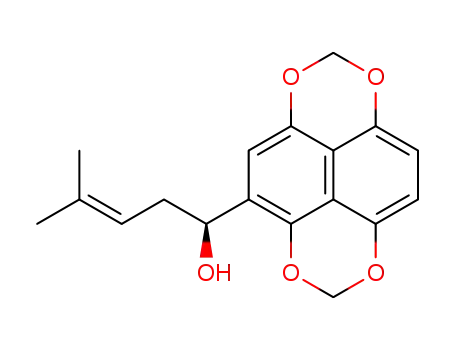
- 206996-07-8
(S)-4-Methyl-1-(1,3,6,8-tetraoxa-pyren-4-yl)-pent-3-en-1-ol

-
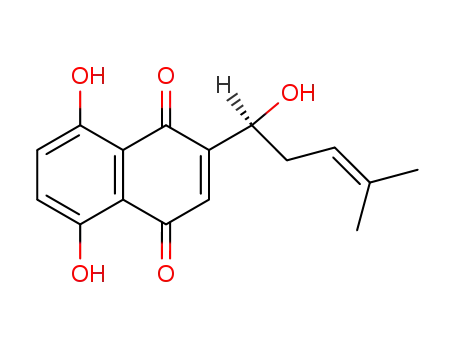
- 517-88-4,517-89-5,11031-58-6,54952-43-1,85921-41-1
(S)-5,8-dihydroxy-2-(1-hydroxy-4-methylpent-3-enyl)naphthalene-1,4-dione
| Conditions | Yield |
|---|---|
|
With lithium perchlorate; copper(II) acetate monohydrate; In water; acetonitrile; for 3h; Electrochemical reaction;
|
85% |
|
With lithium perchlorate; In water; acetonitrile; at 25 ℃; Yield given; anodic oxidation, graphite electrodes, 3V;
|
-
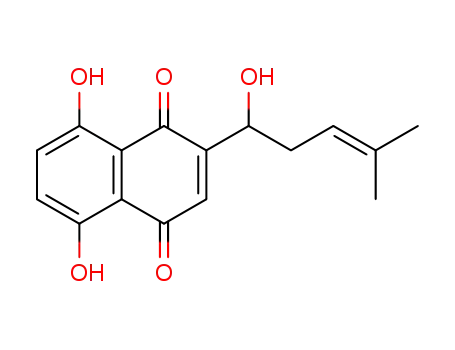
- 517-88-4,517-89-5,11031-58-6,54952-43-1,85921-41-1
shikonin

-
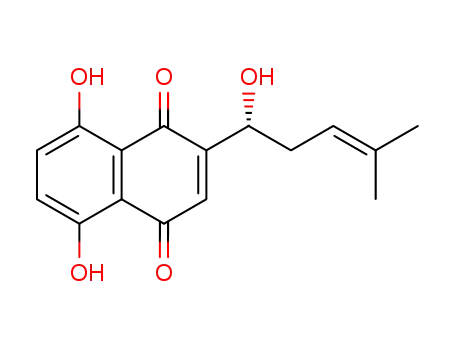
- 517-89-5
shikonin

-

- 517-88-4,517-89-5,11031-58-6,54952-43-1,85921-41-1
(S)-5,8-dihydroxy-2-(1-hydroxy-4-methylpent-3-enyl)naphthalene-1,4-dione
| Conditions | Yield |
|---|---|
|
With Sino-Chiral OD column; In ethanol; Further stages; Resolution of racemate;
|
517-89-5 Upstream products
-
145668-25-3
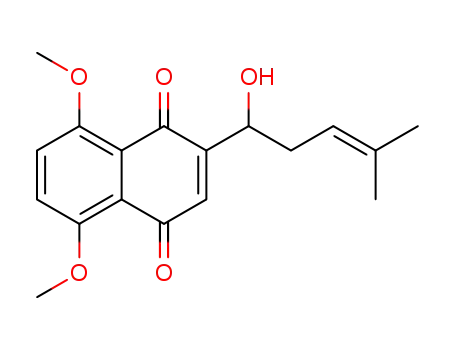
2-(1'-hydroxy-4'-methylpent-3'-en-1'-yl)-5,8-dimethoxy-1,4-naphthoquinone
-
88818-36-4
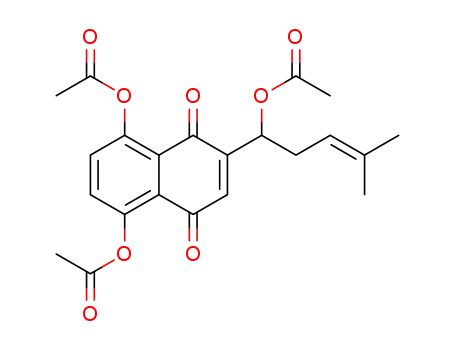
5,8-diacetoxy-2-(1-acetoxy-4-methyl-3-pentenyl)-1,4-naphthoquinone
-
443686-76-8
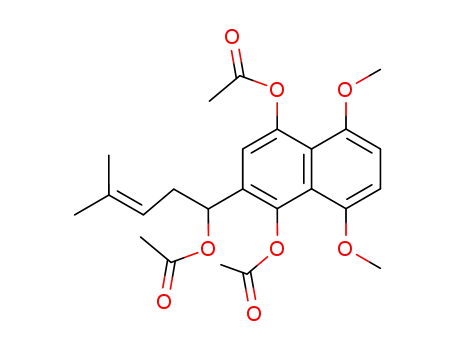
(R,S)-acetic acid 4-acetoxy-3-(1-acetoxy-4-methyl-pent-3-enyl)-5,8-dimethoxynaphthalen-1-yl ester
-
504-85-8
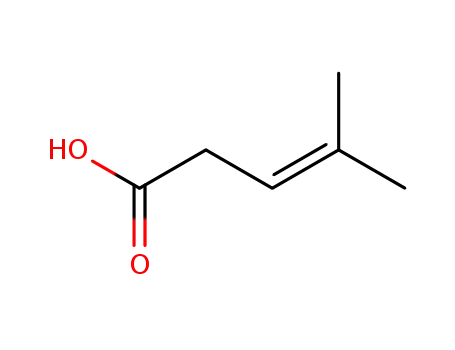
4-methyl-pent-3-enoic acid
517-89-5 Downstream products
-
3724-65-0
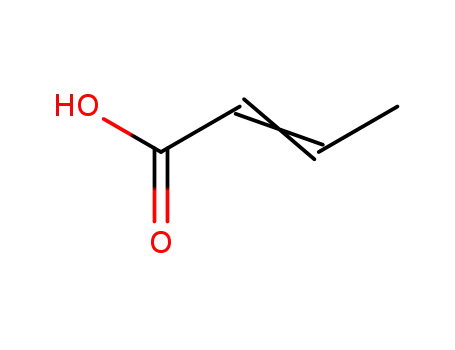
2-butenoic acid
-
84273-03-0
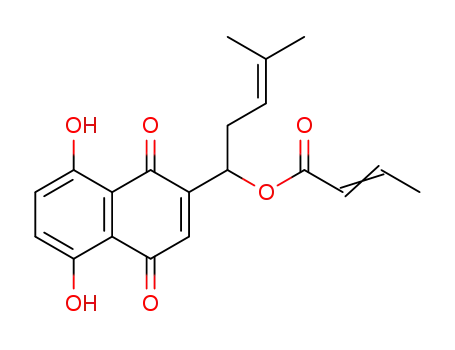
Shikonin Crotonate
-
5162-00-5
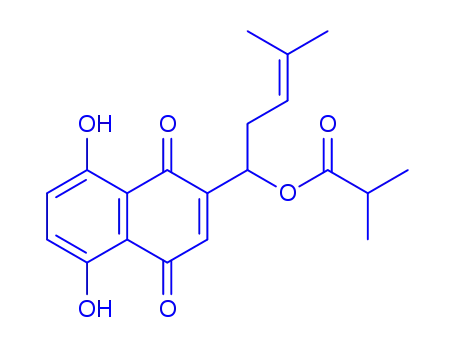
isobutyrylshikonin
-
76549-35-4
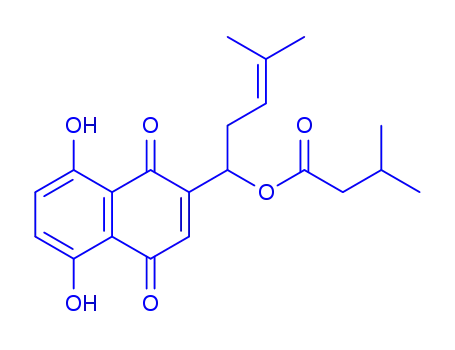
isovalerylshikonin