145-13-1
- Product Name:Pregnenolone
- Molecular Formula:C21H32O2
- Purity:99%
- Molecular Weight:316.484
High Purity Trustworthy Manufacturer Supply Pregnenolone 145-13-1 with Lowest Price
- Molecular Formula:C21H32O2
- Molecular Weight:316.484
- Appearance/Colour:white to off-white powder
- Vapor Pressure:1.02E-09mmHg at 25°C
- Melting Point:188-190 °C
- Refractive Index:1.549
- Boiling Point:443.3 °C at 760 mmHg
- PKA:15.00±0.70(Predicted)
- Flash Point:188.9 °C
- PSA:37.30000
- Density:1.09 g/cm3
- LogP:4.51530
Pregnenolone(Cas 145-13-1) Usage
|
Chemical Description |
Pregnenolone is a steroid hormone involved in the production of other hormones such as estrogen and testosterone. |
|
in vitro |
pregnenolone is the precursor from which almost all of the other steroid hormones are made, including dhea, testosterone, progesterone, the estrogens and cortisol. pregnenolone also operates as a powerful neurosteroid in the brain, which modulates the transmission of messages from neuron to neuron and strongly influencing learning and memory processes [1]. |
|
in vivo |
the ability of pregnenolone sulfate derivatives (pregs) to modulate the age-induced learning impairment was tested in 16- month-old mice using the step-down type of passive avoidance. decreased step-down latency was observed in the passive avoidance task in 16-month-old mice compared to 3-month-old mice, revealing retention deficits in old mice. pretraining injections of pregs dose-dependently improved the 24 h delay retention performances in this task in old mice [1]. |
|
references |
[1] vallée m, mayo w, le moal m. role of pregnenolone, dehydroepiandrosterone and their sulfate esters on learning and memory in cognitive aging. brain res brain res rev. 2001 nov;37(1-3):301-12. |
|
Definition |
ChEBI: A 20-oxo steroid that is pregn-5-ene substituted by a beta-hydroxy group at position 3 and an oxo group at position 20. |
|
General Description |
Pregnenolone is an endogenous steroid hormone involved in the biological synthesis of other hormones. Pregnenolone is monitored as an ancillary test for congenital adrenal hyperplasia (CAH), particularly in situations where diagnosis of 21-hydroxylase and 11-hydroxylase deficiency has been ruled out. This Certified Spiking Solution? is suitable for use in LC-MS/MS testing methods as a starting material in the preparation of calibrators, controls and linearity standards. |
InChI:InChI=1/C21H32O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h4,15-19,23H,5-12H2,1-3H3/t15-,16-,17+,18?,19?,20-,21+/m0/s1
145-13-1 Relevant articles
Synthesis and Biological Evaluation of Vitamin D3 Metabolite 20S,23S-Dihydroxyvitamin D3 and Its 23R Epimer
Lin, Zongtao,Marepally, Srinivasa R.,Ma, Dejian,Kim, Tae-Kang,Oak, Allen Sw.,Myers, Linda K.,Tuckey, Robert C.,Slominski, Andrzej T.,Miller, Duane D.,Li, Wei
, p. 5102 - 5108 (2016)
The vitamin D3 metabolite, 20S,23S-dihyd...
Enzymatic deacetylation of steroids bearing labile functions
Baldessari, Alicia,Maier, Marta S.,Gros, Eduardo G.
, p. 4349 - 4352 (1995)
Lipase from Candida cylindracea and Cand...
1,5-Hydride shift in Wolff-Kishner reduction of (20R)-3β,20, 26-trihydroxy-27-norcholest-5-en-22-one: Synthetic, quantum chemical, and NMR studies
Szendi, Zsuzsanna,Forgo, Peter,Tasi, Gyula,Boecskei, Zsolt,Nyerges, Levente,Sweet, Frederick
, p. 31 - 38 (2002)
Heating (20R)-3β,20,26-trihydroxy-27-nor...
Ile351, Leu355 and Ile461 residues are essential for catalytic activity of bovine cytochrome P450scc (CYP11A1)
Glyakina, Anna V.,Strizhov, Nicolai I.,Karpov, Mikhail V.,Dovidchenko, Nikita V.,Matkarimov, Bakhyt T.,Isaeva, Ludmila V.,Efimova, Vera S.,Rubtsov, Mikhail A.,Novikova, Ludmila A.,Donova, Marina V.,Galzitskaya, Oxana V.
, p. 80 - 90 (2019)
Cytochrome P450scc (CYP11A1) is a mammal...
Discovery of novel steroidal-chalcone hybrids with potent and selective activity against triple-negative breast cancer
Bai, Chengfeng,Hou, Qiangqiang,Lin, Xin,Lu, Xiang,Luo, Guoshun,Wei, Hanlin,Xiang, Hua
, (2020)
A series of novel steroidal-chalcone der...
3-Methoxybenzidine: A potent inhibitor of cholesterol side chain cleavage cytochrome P-450
Duval,Vickery
, p. 473 - 481 (1980)
-
A new mild PTSA-catalyzed method for sulfate ester hydrolysis and acid-catalyzed rearrangement of 12-acetyl-diene-11-ol tetracyclic triterpenoids involving an angular methyl migration
Singh
, p. 6973 - 6976 (2000)
Tetracyclic triterpenoids containing the...
Inhibition of bovine adrenocortical cytochrome P-450scc by 3,3'-dimethoxybenzidine
Duval,Vickery
, p. 91 - 101 (1981)
The effect of 3,3'-dimethoxybenzidine (o...
Mild deprotection of steroid esters by bis(tributyltin)oxide
Perez, Marina G.,Maier, Marta S.
, p. 3311 - 3314 (1995)
Bis(tributyltin)oxide (BBTO) has been ut...
Human placental cholesterol side-chain cleavage: enzymatic synthesis of (22R)-20α,22-dihydroxycholesterol
Tuckey, Robert C.,Cameron, Kathryn J.
, p. 230 - 233 (1993)
(22R)-20α,22-Dihydroxycholesterol is the...
-
Miescher,Kaegi
, p. 184 (1939)
-
-
Fieser,Huang-Minlon
, p. 1840,1842 (1949)
-
Proline-promoted dehydroxylation of α-ketols
Mostinski, Yelena,Lankri, David,Konovalov, Yana,Nataf, Riva,Tsvelikhovsky, Dmitry
, p. 9345 - 9350 (2019)
A new single-step proline-potassium acet...
Cholesterol side-chain cleavage activity in human placenta and bovine adrenals: An one-step method for separation of pregnenolone formed in vitro
Pasanen,Pelkonen
, p. 517 - 527 (1984)
Cholesterol side-chain cleavage (CSCC) a...
-
Butenandt,Fleischer
, p. 96,100 (1937)
-
Microbial hydroxylation of pregnenolone derivatives
Choudhary, Muhammad Iqbal,Batool, Iffat,Shah, Syed Adnan Ali,Nawaz, Sarfraz Ahmad,Atta-ur-Rahman
, p. 1455 - 1459 (2005)
Pregnenolone (1) and pregnenolone acetat...
FRAGMENTATION OF 16α,17α-CYCLOBUTENOPREGNANE
Kamernitskii, A.V.,Ignatov, V.N.,Levina, I.S.,Kulikova, L.E.
, p. 2399 - 2401 (1984)
-
From organocatalysed desilylations to high-yielding benzylidenations of electron-deficient benzaldehydes
Niu, Qun,Xing, Linlin,Li, Chunbao
, p. 358 - 364 (2017)
A new type of organoprecatalyst (MeSCH2C...
Stereoselective synthesis of (22R,23R,24S)-3β-Hydroxy-5-ene-22,23-dihydroxy-24-methyl-cholestane: A Brassinolide Intermediate from 16-Dehydropregnenolone Acetate
Hazra, Braja G.,Joshi, Padmakar L.,Bahule, Bharat B.,Argade, Narshinha P.,Pore, Vandana S.,Chordia, Mahendra D.
, p. 2523 - 2532 (1994)
A new synthesis of the important aldehyd...
A Dual Role Reductase from Phytosterols Catabolism Enables the Efficient Production of Valuable Steroid Precursors
Peng, Haidong,Wang, Yaya,Jiang, Kai,Chen, Xinru,Zhang, Wenlu,Zhang, Yanan,Deng, Zixin,Qu, Xudong
supporting information, p. 5414 - 5420 (2021/02/05)
4-Androstenedione (4-AD) and progesteron...
Steroid compound as well as preparation method and application thereof (by machine translation)
-
, (2020/10/29)
The compounds have the structure shown i...
ENABLING CHOLESTEROL CATABOLISM IN HUMAN CELLS
-
Paragraph 0029, (2020/07/05)
Compositions, methods, and systems for m...
145-13-1 Process route
-
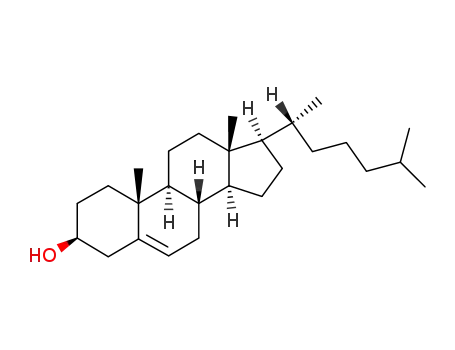
-
57-88-5
cholesterol

-
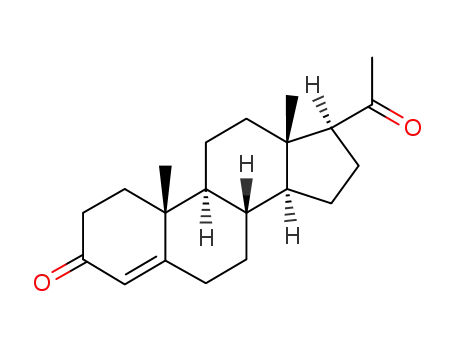
-
57-83-0
Progesterone

-
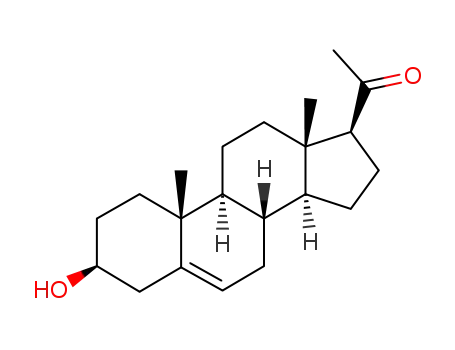
-
145-13-1
Pregnenolone

-
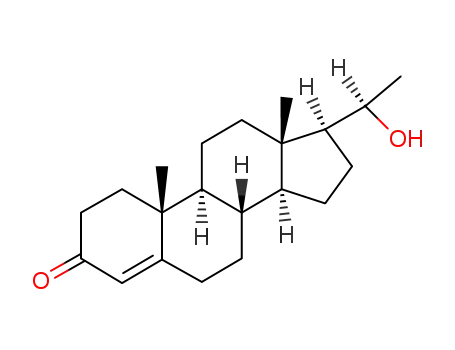
-
145-15-3
20α-hydroxyprogesterone

-
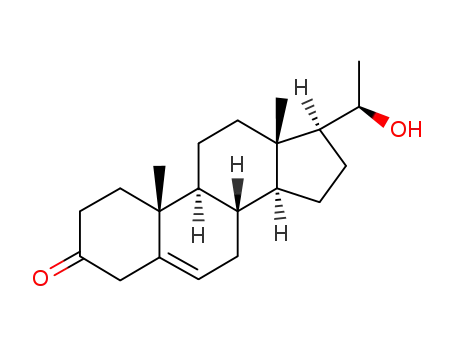
-
566-64-3
(20R)-20-Hydroxy-5-pregnen-3-on

-
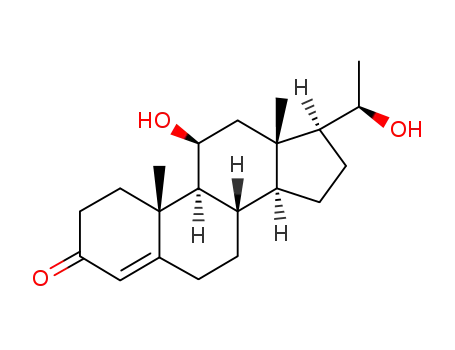
-
2640-53-1
11β-hydroxy-20α-dihydroprogesterone
| Conditions | Yield |
|---|---|
|
With
Ham's F-10 medium; mouse adrenal Y-1 cells;
at 37 ℃;
for 60h;
Product distribution;
metabolism, 14C-labeled, further stimulation by ACTH;
|
|
|
With
calf serum horse serum; Ham's F-10 medium; MEN medium; mouse adrenal Y-1 cells transformed by simian adenovirus SA-7;
at 37 ℃;
for 60h;
Product distribution;
|
-

-
57-83-0
Progesterone

-
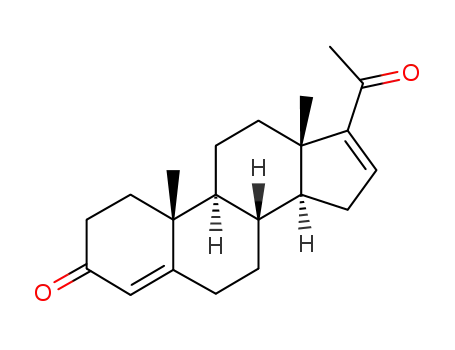
-
1096-38-4
16-dehydroprogesterone

-
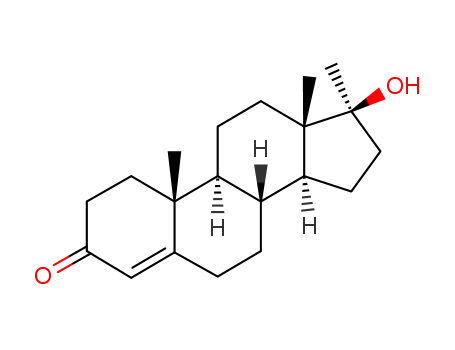
-
58-18-4
17-methyltestosterone

-

-
145-13-1
Pregnenolone

-
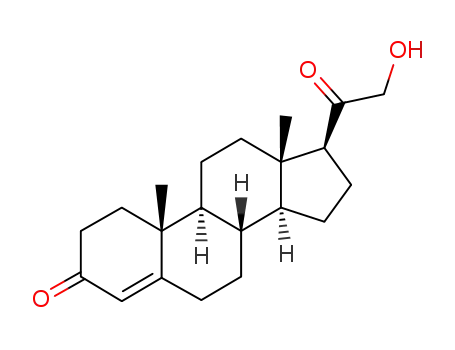
-
64-85-7
21-Hydroxyprogesterone

-
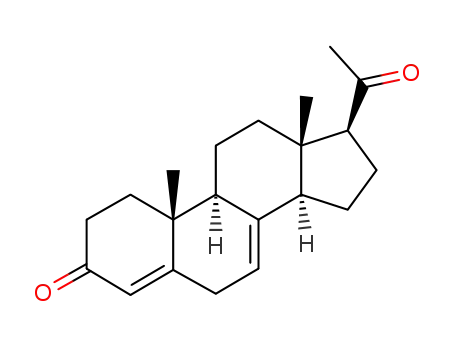
-
17398-60-6
pregna-4,7-diene-3,20-dione

-
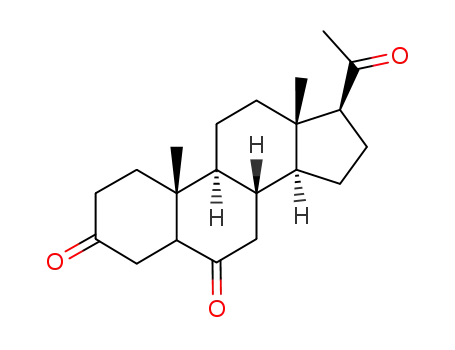
-
30802-24-5
pregnane-3,6,20-trione

-
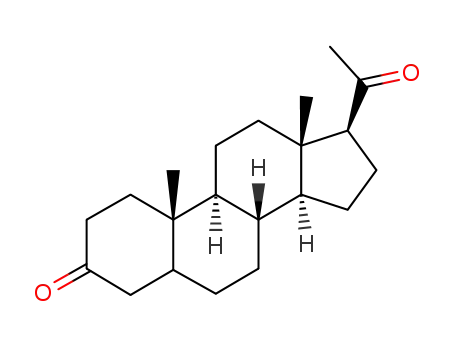
-
7350-00-7
pregnane-3,20-dione
| Conditions | Yield |
|---|---|
|
With
Hortaea werneckii B-763 at all growth phases;
In
YNB growth medium; N,N-dimethyl-formamide;
at 28 ℃;
for 24h;
Enzymatic reaction;
|
145-13-1 Upstream products
-
1162-53-4
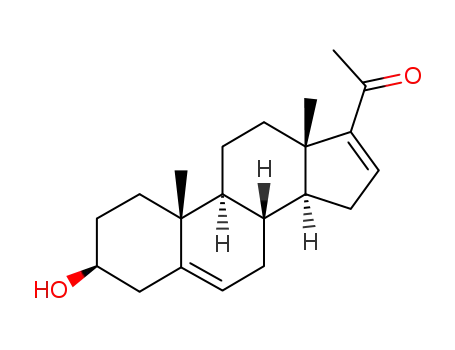
16-dehydropregnenolone
-
917-64-6
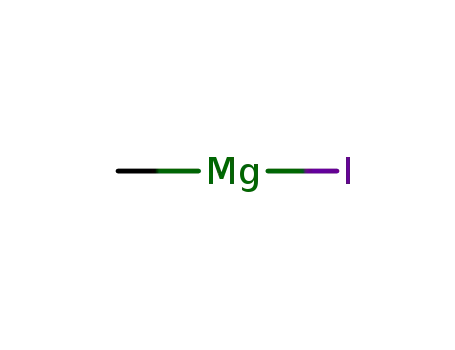
methyl magnesium iodide
-
57764-90-6
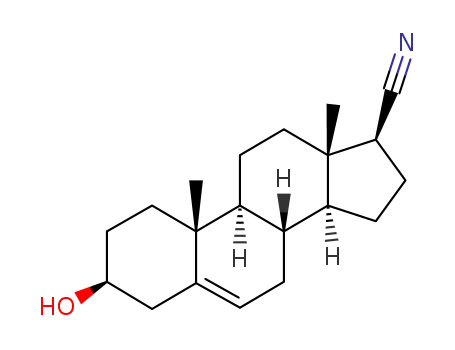
3β-hydroxy-androstene-(5)-carboxylic acid-(17β)-nitrile
-
996-82-7
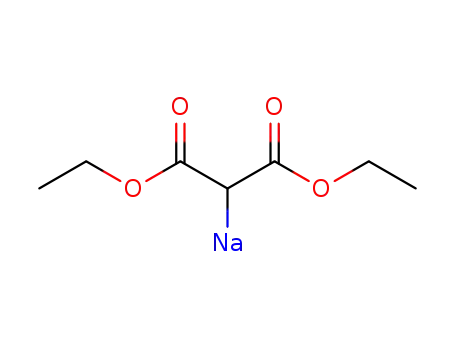
sodium diethylmalonate
145-13-1 Downstream products
-
566-65-4
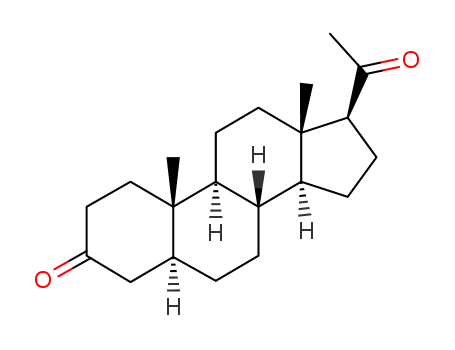
dihydroprogesterone
-
1232-18-4
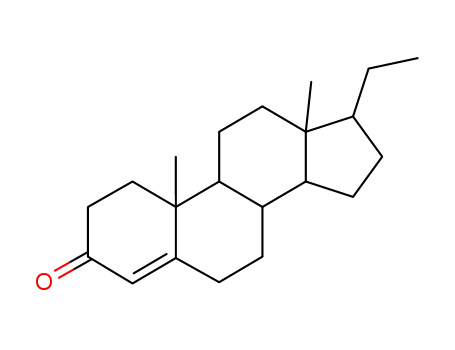
17-Ethyl-10,13-dimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-3-one
-
23328-05-4
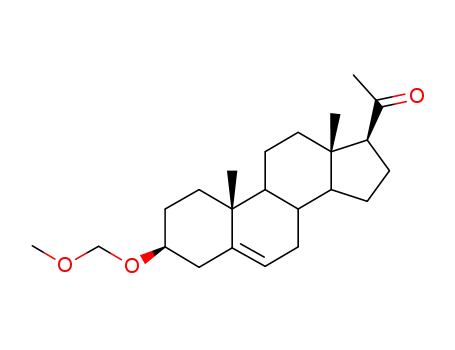
3β-methoxymethoxy-5-pregnen-20-one
-
32207-28-6
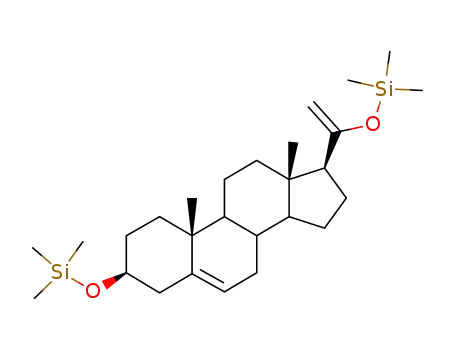
Pregnenolon-20-en-20-ol-trimethylsilyl-ether