69004-03-1
- Product Name:Toltrazuril anticoccidial
- Molecular Formula:C18H14F3N3O4S
- Purity:99%
- Molecular Weight:425.388
Quality Factory Supply High Purity 99% Toltrazuril anticoccidial 69004-03-1 with Efficient Shipping
- Molecular Formula:C18H14F3N3O4S
- Molecular Weight:425.388
- Appearance/Colour:Powder
- Melting Point:194-196°C
- Refractive Index:1.64
- PKA:6.47±0.20(Predicted)
- PSA:111.39000
- Density:1.54 g/cm3
- LogP:2.93710
Toltrazuril anticoccidial(Cas 69004-03-1) Usage
|
Physical properties |
Toltrazuril is white or off-white color crystalline powder, and odorless is molten in the methanol part omitted at ethyl acetate, dichloromethane, Polyethylene Glycol, α-adjoin in the organic solvents such as pyrrolidone, propylene glycol and dissolve, and is insoluble in water. |
|
Uses |
Toltrazuril is a triazinetrione derivative administered orally in the drinking water for the treatment of coccidiosis in chickens and turkeys. The recommended dose and duration of treatment for chickens and turkeys is 7 mg/kg bodyweight per day for two consecutive days. |
|
Environmental Fate |
The metabolite of toltrazuril, toltrazuril sulfone (ponazuril) is a persistent (half-life >1 year) and mobile compound and has adverse effects on both the growth and emergence of plants. Given the persistent properties of ponazuril repeated spreading of manure from treated animals may lead to an accumulation in the soil and consequently a risk to plants. The accumulation of ponazuril in soil together with its mobility also leads to a risk of leaching to groundwater. |
InChI:InChI=1/C18H14F3N3O4S/c1-10-9-11(24-16(26)22-15(25)23(2)17(24)27)3-8-14(10)28-12-4-6-13(7-5-12)29-18(19,20)21/h3-9H,1-2H3,(H,22,25,26)
69004-03-1 Relevant articles
Method for synthesizing anticoccidial drug toltrazuril for animals
-
Paragraph 0047-0053, (2020/06/05)
The invention discloses a method for syn...
Field evaluation of anticoccidial efficacy: a novel approach demonstrates reduced efficacy of toltrazuril against ovine Eimeria spp. in Norway
Ane Odden a, Matthew J. Denwood b, Snorre Stuen a, Lucy J. Robertson c, Antonio Ruiz d, Inger Sofie Hamnes e, Lisbeth Hektoen f 1, Heidi L. Enemark e
International Journal for Parasitology: Drugs and Drug Resistance Volume 8, Issue 2, August 2018, Pages 304-311
Ovine Eimeria spp. infections cause reduced welfare, increased mortality, and substantial economic losses, and anticoccidials are crucial for their control. Recent reports of toltrazuril resistance in pigs, and anecdotal reports of reduced anticoccidial efficacy in lambs, necessitate evaluation of anticoccidial efficacy.
69004-03-1 Process route
-
![3-methyl-1-[3-methyl-4-(4-(trifluoromethylsulfanyl)phenoxy)phenyl]biuret](/upload/2024/5/0d4f3104-f20c-4a8e-b578-a2955be52af9.png)
- 106310-18-3
3-methyl-1-[3-methyl-4-(4-(trifluoromethylsulfanyl)phenoxy)phenyl]biuret

-
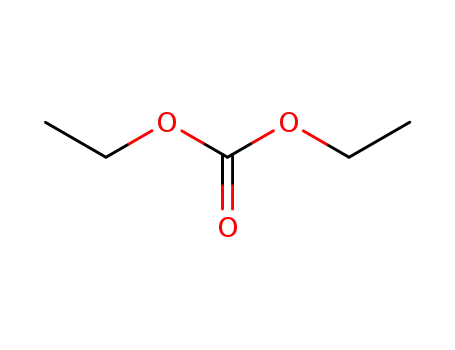
- 105-58-8,76619-83-5
Diethyl carbonate

-
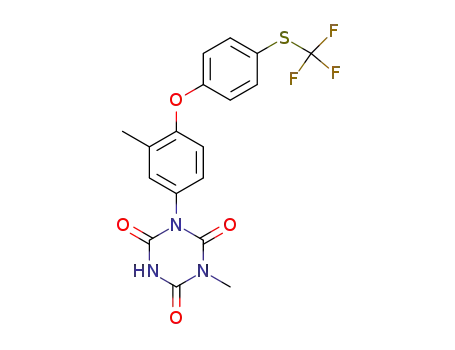
- 69004-03-1
toltrazuril
| Conditions | Yield |
|---|---|
|
3-methyl-1-[3-methyl-4-(4-(trifluoromethylsulfanyl)phenoxy)phenyl]biuret; Diethyl carbonate; With sodium methylate; In toluene; at 50 ℃; for 4h;
With acetic acid; In water; toluene; at 70 ℃; pH=7; Reagent/catalyst; Solvent; Temperature;
|
81% |
|
With sodium methylate; In water;
|
-
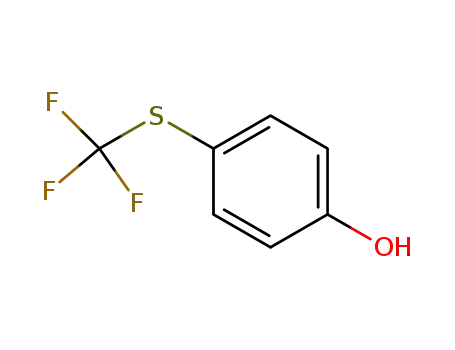
- 461-84-7
(4-trifluoromethylthio)phenol

-
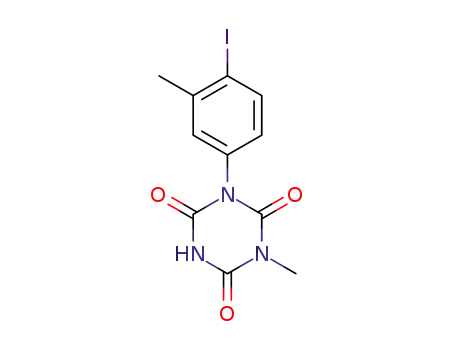
-
1-(4-iodo-3-methylphenyl)-3-methyl-1,3,5-triazine-2,4,6-trione

-

- 69004-03-1
toltrazuril
| Conditions | Yield |
|---|---|
|
With potassium phosphate; copper(l) iodide; N-(2-phenylphenyl)-N′-benzyl oxalamide; In acetonitrile; at 90 ℃; for 24h; Inert atmosphere;
|
80.6% |
69004-03-1 Upstream products
-
106310-17-2
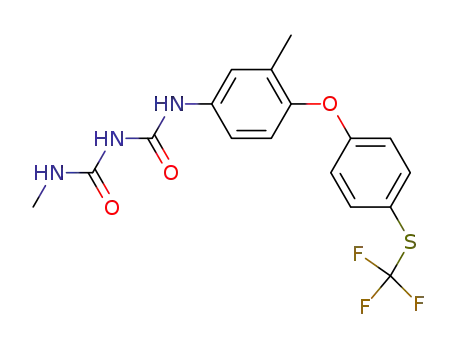
N-methyl-N'-[3-methyl-4-[4-[(trifluoromethyl)thio]phenoxy]phenyl] imidodicarbonic diamide
-
105-58-8

Diethyl carbonate
-
106310-18-3
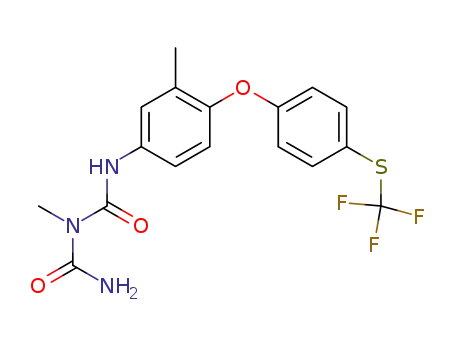
3-methyl-1-[3-methyl-4-(4-(trifluoromethylsulfanyl)phenoxy)phenyl]biuret
-
94155-78-9
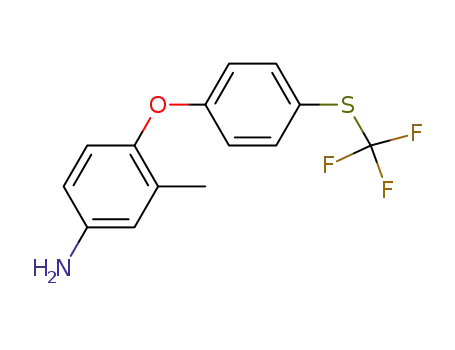
3-methyl-4-{4-[(trifluoromethyl)sulfanyl]phenoxy}aniline