73231-34-2
- Product Name:Florfenicol
- Molecular Formula:C12H14Cl2FNO4S
- Purity:99%
- Molecular Weight:358.218
Buy High Quality Reputable Manufacturer Supply Florfenicol 73231-34-2 with Cheapest Price
- Molecular Formula:C12H14Cl2FNO4S
- Molecular Weight:358.218
- Appearance/Colour:White crystalline powder
- Vapor Pressure:4.05E-12mmHg at 25°C
- Melting Point:153 °C
- Refractive Index:1.575
- Boiling Point:617.5 °C at 760 mmHg
- PKA:10.73±0.46(Predicted)
- Flash Point:327.3 °C
- PSA:91.85000
- Density:1.451 g/cm3
- LogP:2.70550
Florfenicol(Cas 73231-34-2) Usage
|
Veterinary antibiotics |
Florfenicol is currently a commonly used veterinary antibiotic with a broad antibacterial spectrum and a strong antibacterial effect as well as a low the minimum inhibitory concentration (MIC). The antibacterial of florfenicol is about 15-20 times as high as that of chloramphenicol and the thiamphenicol. After administration through feed for 60 minutes, the drug concentration in the tissue can reach peak which can quickly control the disease with having characteristics such as being safe, non-toxic, no residue, and no risk for triggering aplastic anemia. Therefore, it is quite suitable for large-scale farms application. It is mainly used for the treatment of bovine respiratory disease caused by Pasteurella and Haemophilus. It has good efficacy in treating the cattle footrot disease cause by Fusobacterium, and can be also used for treating the infection diseases of pigs and chickens caused by sensitive strains as well as the bacterial disease of fish. It is not easy for bacteria to evolve resistance against florfenicol: Because it has a fluorine atom which replaces the hydroxyl group on the molecular structure of thiamphenicol, thus effectively solving the drug resistant issue of chloramphenicol, and thiamphenicol. Bacteria which are resistant to thiamphenicol, chloramphenicol, amoxicillin, and quinolone remain sensitive to the chemicals. Since chloramphenicol can result in severe adverse reactions such as aplastic anemia and immune suppression, it is banned from being applied in food animal production. Studies have demonstrated that: the major group in the chemical structure of chloramphenicol which causes aplastic anemia is para-nitro in the aromatic ring. Instead, florfenicol has the CH3SO4 which replaces the NO2 groups so that its chemical structure has been changed. In this case, its application to animal will not cause any adverse reactions such as aplastic anemia. Therefore, it is approved for application in more than a dozen of countries such as Japan, Mexico and China. Florfenicol is characterized by: a broad spectrum antibiotic; Salmonella, Escherichia coli, Proteus, Haemophilus, Actinobacillus pleuropneumoniae, Mycoplasma hyopneumoniae, Streptococcus Suis, swine Pasteurella, Bordetclla bronchiseptica, and Staphylococcus aureus are all sensitive to it. The drug is easy for absorbing and is widely distributed in the body and is quick-acting and long-acting formulations with no potential risk of causing aplastic anemia risks and thus having a better security. In addition, it has an affordable price which is cheaper than other drugs for prevention and treatment of respiratory diseases such as tiamulin, tilmicosin, and azithromycin. The cost of medication is easy for users to accept. Because of all these excellent characteristics, domestic florfenicol is widely applied and has currently become the first-choice drug for prevention and treatment of livestock respiratory diseases and gastrointestinal bacterial infection diseases. Florfenicol is not suitable to be applied in combination with quinolones, penicillins, and cephalosporins, and it has no compatibility with ampicillin, sulfonamides, and furans. The above information is edited by the lookchem of Dai Xiongfeng. |
|
Pharmacological effects |
Florfenicol binds tightly with the 50S subunit of the bacterial ribosome and block the transpeptidation reaction mediated by peptidyl transferase, and thereby suppressing the elongation process of the peptide chain; meanwhile selectively acts on the bacterial 70S ribosome receptors and changes, interferes with the function of peptidyl transferase enzyme. Therefore, it interferes with the bacterial protein synthesis based on dual mechanism. |
|
Indications |
Florfenicol can strongly interfere with the bacterial protein synthesis. It has a rapid absorption rate and is widely distributed in the body; it has long half-life without side effects such as aplastic anemia; not easy for bacteria to evolve drug resistance, and has no cross-resistance. Florfenicol can be used for the treatment of the systemic infection of livestock and aquatic animals, and has significant efficacy on treating respiratory infections and intestinal infections. Poultry: Escherichia coli disease, salmonellosis, infectious rhinitis, chronic respiratory disease, duck plague and other kinds of mixed infections caused by susceptible strains. Animals: Mixed infections such as infectious pleurisy, asthma, streptococcal disease, Escherichia coli disease, salmonellosis, contagious pleuropneumonia, enzootic pneumonia, paratyphoid piglets yellow white diarrhea, edema disease, atrophic rhinitis, lung epidemic, red white diarrhea piglets, and agalactia syndrome occurred in animals such as pigs, cows, and sheep. Crab: appendages ulcer disease, yellow gills, rotten gill, red legs and red fluorescent body inflammatory disease syndrome. Turtle: red neck disease, furunculosis, shot-hole disease, skin fester disease, inflammatory bowel disease, mumps, and bacterial sepsis. Frogs: cataract syndrome, ascites disease, sepsis, enteritis. Fish: enteritis disease, ascites disease, vibriosis, Edward coli disease. Eel: debonding septicemia (unique effect), Edward coli disease, red skin disease, inflammatory bowel disease. |
|
Definition |
ChEBI: Florfenicol is a carboxamide that is the N-dichloroacetyl derivative of (1R,2S)-2-amino-3-fluoro-1-[4-(methanesulfonyl)phenyl]propan-1-ol. A synthetic veterinary antibiotic that is used for treatment of bovine respiratory disease and foot rot; also used in aquaculture. It has a role as an antimicrobial agent. It is a sulfone, a secondary alcohol, an organofluorine compound, an organochlorine compound and a secondary carboxamide. It is functionally related to a dichloroacetic acid. |
|
Preparation |
Florfenicol is synthesised from thiamphenicol by replacing the 3-hydroxy group with fluorine, first synthesised at Schering in 1980. By replacing the hydroxy group, it was rationalised that chloramphenicol resistance via chloramphenicol acetyltransferase could be eliminated. Florfenicol is a broad spectrum antibiotic with good activity against Gram negative and anaerobic bacteria. It acts by binding to the 23S sub-unit of the 50S ribosome, inhibiting protein synthesis. Florfenicol has been extensively studied with over 400 literature citations. |
|
Veterinary Drugs and Treatments |
The drug is approved for use in cattle only (in the USA) for the treatment of bovine respiratory disease (BRD) associated with Pasteurella haemolytica, Pasteurella multocida, and Haemophilus somnus. Because florfenicol has activity against a wide range of microorganisms (e.g., Mycoplasma), it may be useful for treating other infections in cattle (or other species) as well, but specific data is limited. |
InChI:InChI=1/C12H14Cl2FNO2S/c1-19-8-4-2-7(3-5-8)10(17)9(6-15)16-12(18)11(13)14/h2-5,9-11,17H,6H2,1H3,(H,16,18)
73231-34-2 Relevant articles
Catalytic Syn-Selective Nitroaldol Approach to Amphenicol Antibiotics: Evolution of a Unified Asymmetric Synthesis of (-)-Chloramphenicol, (-)-Azidamphenicol, (+)-Thiamphenicol, and (+)-Florfenicol
Chen, Fener,Cheng, Dang,Huang, Huashan,Jiang, Meifen,Liu, Minjie,Qu, Hongmin,Xia, Yingqi,Xiong, Tong,Zhang, Yan
, p. 11557 - 11570 (2021/09/02)
A unified strategy for an efficient and ...
METHODS FOR PREPARING FLORFENIOL AND INTERMEDIATE THEREOF
-
, (2021/07/02)
The present invention discloses a method...
Synthesis method of florfenicol
-
, (2021/03/24)
The invention discloses a synthesis meth...
Method for continuously preparing florfenicol based on micro-reaction system
-
Paragraph 0053-0054; 0057-0061; 0065-0068; 0072-0075; ..., (2021/07/24)
The invention provides a method for cont...
73231-34-2 Process route
-
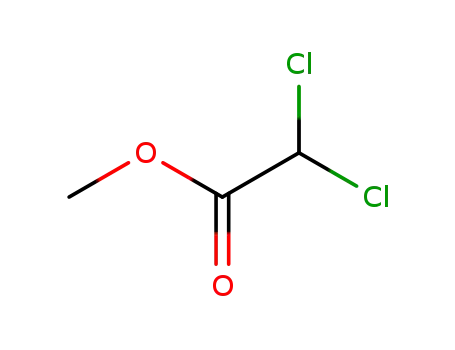
-
116-54-1
dichloroacetic acid methyl ester

-
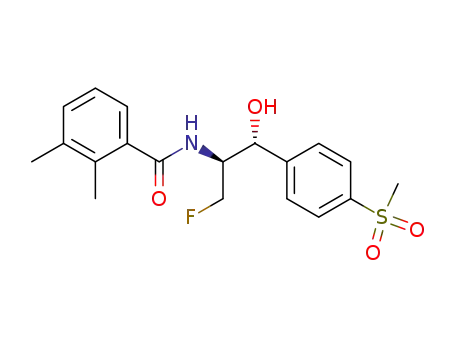
-
108656-27-5
(1R,2S)-2-(2,3-dimethylbenzoyl)amino-3-fluoro-1-<4-(methylsulphonyl)phenyl>-1-propanol

-
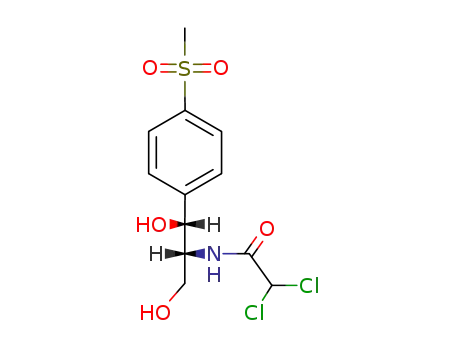
-
15318-45-3,90-91-5
thiamphenicol

-
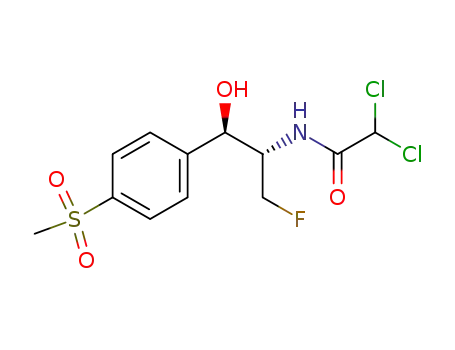
-
73231-34-2
Florfenicol
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; triethylamine;
Yield given. Multistep reaction. Yields of byproduct given;
1.) 120 deg C, 5 h, 2.) 80 deg C, 2 h;
|
-

-
116-54-1
dichloroacetic acid methyl ester

-
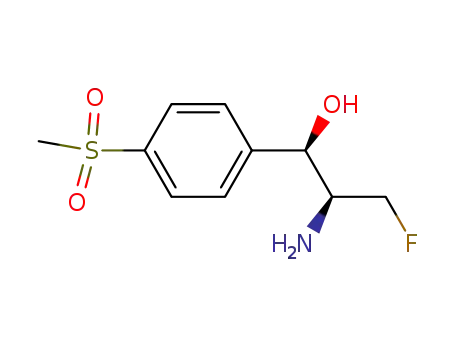
-
76639-93-5,105182-37-4,118015-48-8
(1R,2S)-2-amino-3-fluoro-1-<4-(methylsulphonyl)phenyl>-1-propanol

-

-
73231-34-2
Florfenicol
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
methanol;
at 20 ℃;
|
96% |
|
With
triethylamine;
In
methanol;
for 18h;
Ambient temperature;
|
84% |
|
With
triethylamine;
for 3.5h;
Yield given;
Heating;
|
|
|
With
sodium carbonate;
In
methanol;
at 60 - 65 ℃;
for 2h;
|
|
|
With
triethylamine;
In
methanol;
at 20 - 25 ℃;
for 12 - 16h;
Product distribution / selectivity;
|
|
|
With
triethylamine;
In
methanol;
at 20 - 25 ℃;
|
|
|
With
triethylamine;
In
methanol;
for 3h;
Inert atmosphere;
Reflux;
|
1.04 g |
|
With
triethylamine;
In
methanol;
at 45 ℃;
|
|
|
With
triethylamine;
In
methanol;
at 50 ℃;
for 12h;
|
6.5 g |
|
With
triethylamine;
In
methanol;
at 40 ℃;
for 6h;
|
3.4 g |
|
With
triethylamine;
In
methanol;
at 50 ℃;
for 10h;
|
7.1 g |
73231-34-2 Upstream products
-
116-54-1

dichloroacetic acid methyl ester
-
76639-93-5

(1R,2S)-2-amino-3-fluoro-1-<4-(methylsulphonyl)phenyl>-1-propanol
-
108656-27-5

(1R,2S)-2-(2,3-dimethylbenzoyl)amino-3-fluoro-1-<4-(methylsulphonyl)phenyl>-1-propanol
-
4302-89-0
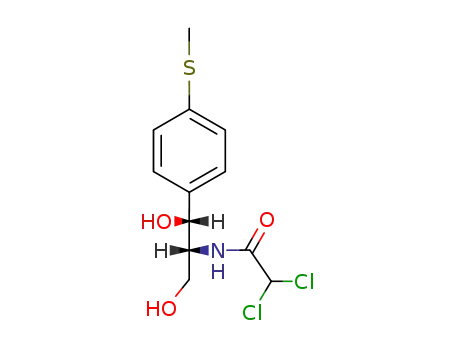
(1R,2R)-2-(2,2-dichloro-acetylamino)-1-(4-methylsulfanyl-phenyl)-propane-1,3-diol
73231-34-2 Downstream products
-
76639-93-5

(1R,2S)-2-amino-3-fluoro-1-<4-(methylsulphonyl)phenyl>-1-propanol
-
864529-18-0
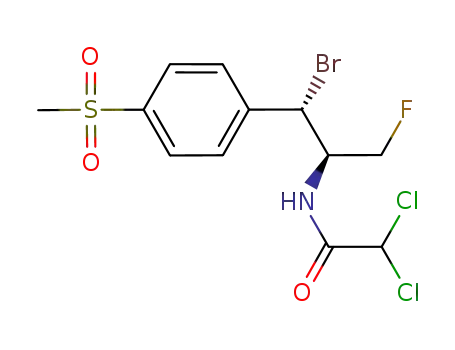
C12H13BrCl2FNO3S
-
1073342-33-2
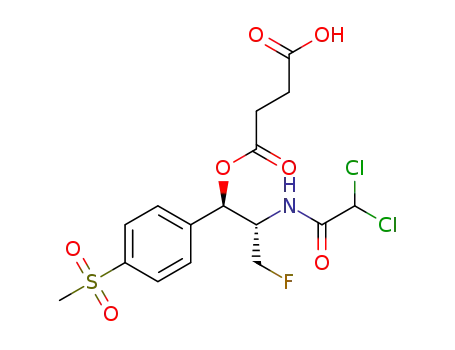
4-((1R,2S)-2-(2,2-dichloroacetamido)-3-fluoro-1-(4-(methylsulfonyl)phenyl)propoxy)-4-oxobutanoic acid
-
1450713-53-7
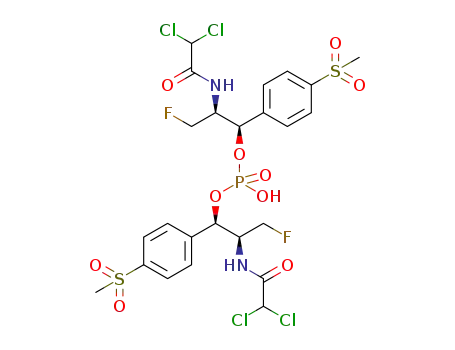
bis((1R,2S)-2-(2,2-dichloroacetamido)-3-fluoro-1-(4-(methylsulfonyl)phenyl)propyl) hydrogen phosphate