43210-67-9
- Product Name:Fenbendazole
- Molecular Formula:C15H13N3O2S
- Purity:99%
- Molecular Weight:299.353
99% Pure Factory Supply Fenbendazole 43210-67-9 Customized Supply
- Molecular Formula:C15H13N3O2S
- Molecular Weight:299.353
- Appearance/Colour:Off-white solid
- Melting Point:233 °C
- Refractive Index:1.709
- PKA:10.80±0.10(Predicted)
- PSA:92.31000
- Density:1.4 g/cm3
- LogP:3.96540
Fenbendazole(Cas 43210-67-9) Usage
|
Benzimidazole anthelmintics |
Fenbendazole is a benzimidazole anthelmintic with a broad spectrum,high efficiency and low toxicity ,in the structure,it has a strong affinity with the parasite tubulin ,by influencing cell transport and energy metabolism,it plays a role in preventing the polymerization of micro tubes, which ultimately destroys the integrity of parasite cells and energy transmission. Fenbendazole is used to drive and to kill the animal gastrointestinal parasites, it not only has a highly anthelmintic activity on animal gastrointestinal roundworms, hookworms, whipworms, some tapeworms and nematodes , but also has a preferred effect on the part of the bronchial tree and lung parasites (Aelurostrongylus abstrutus and lung fluke) . |
|
Aofendazuo |
Aofendazuo is a benzimidazole anthelmintic, also known as the Austrian phenol metronidazole, oxfendazole, benzene etomidate sulfoxide, benzene sulfur oxygen imidazole ,it is the sulfoxide form of Fenbendazole , white or off-white powder at room temperature, slight special smell, it can damage the microtubules of the worms epithelial cells of the gastrointestinal tract, and inhibit the worms uptake of glucose from the intestine. Aofendazuo is used for the treatment and control of gastrointestinal adults and larvae, it has a good effect on the treatment of in vivo roundworms and lung worms in pigs and sheep , including Oster nematodes (Ostertagia), Haemonchus , Trichostrongylus, Nematodirus , Cooper nematode (Cooperia), capillary nematodes (Capillaria), toothed Oesophagostomum (Oesophagostomum), Chabertia, Trichuris nematodes form (Trichuris) and dictyocaulasis ( Dictyocaulus) and so on. The above information is edited by the lookchem of Tian Ye. |
|
Production method |
5-chloro-2-nitroaniline reacts with benzene thiol, to thereby obtain 5-phenylthio-2-nitroaniline in 91% yield.Generate 3-phenylthio-o-phenylenediamine by ferrous sulfate-iron reduction in 90% yield. Finally, cyclize it with the S-methyl-melamine Methyl to obtain fenbendazole. |
|
Definition |
ChEBI: Fenbendazole is a member of the class of benzimidazoles that is 1H-benzimidazole which is substituted at positons 2 and 5 by (methoxycarbonyl)amino and phenylsulfanediyl groups, respectively. A broad-spectrum anthelmintic, it is used, particularly in veterinary medicine, for the treatment of nematodal infections. It has a role as an antinematodal drug. It is a member of benzimidazoles, a carbamate ester and an aryl sulfide. |
|
Manufacturing Process |
20.9 g of S-methyl-thiourea were dissolved in 27 ml of water with 13.5 ml of chloroformic acid methyl ester. Then, 45.7 ml of 25% sodium hydroxide solution were added dropwise, while stirring, at a temperature of 5°C to 10°C. After having stirred for 20 minutes, the reaction mixture was combined with 27 ml of glacial acetic acid, 100 ml of water and 29 g of 3,4-diaminodiphenyl- thioether. Stirring was continued for 90 minutes at a temperature of 85°C, during which time methyl-mercaptan was separated. After having allowed the whole to cool and stand overnight, the 5-phenylmercaptobenzimidazole- 2-methyl-carbamate that had formed was filtered off with suction. After recrystallization from a mixture of glacial acetic acid and methanol, 14 g of 4-phenylmercapto-benzimidazole-2-methyl-carbamate melting at 233°C were obtained. |
|
Brand name |
Panacur (Hoechst-Roussel). |
|
Therapeutic Function |
Anthelmintic |
|
General Description |
Fenbendazole is a thio substituted benzimidazole, which belongs to the group of anthelmintics. It can be widely used in veterinary medicine particularly, in the treatment of helminth infections. |
|
Veterinary Drugs and Treatments |
Fenbendazole is indicated (labeled) for the removal of the following parasites in dogs: ascarids (Toxocara canis, T. leonina), Hookworms (Ancylostoma caninum, Uncinaria stenocephala), whipworms (Trichuris vulpis), and tapeworms (Taenia pisiformis). It is not effective against Dipylidium caninum. Fenbendazole has also been used clinically to treat Capillaria aerophilia, Filaroides hirthi, and Paragonimus kellicotti infections in dogs.Fenbendazole is indicated (labeled) for the removal of the following parasites in cattle: Adult forms of: Haemonchus contortus, Ostertagia ostertagi, Trichostrongylus axei, Bunostomum phlebotomum, Nematodirus helvetianus, Cooperia spp., Trichostrongylus colubriformis, Oesophagostomum radiatum, and Dictyocaulus vivaparus. It is also effective against most immature stages of the above listed parasites. Although not approved, it has good activity against Moniezia spp., and arrested 4th stage forms of Ostertagia ostertagi. Fenbendazole is indicated (labeled) for the removal of the following parasites in horses: large strongyles (S. edentatus, S. equinus, S. vulgaris), small strongyles (Cyathostomum spp., Cylicocylus spp., Cylicostephanus spp., Triodontophorus spp.), and pinworms (Oxyuris equi).Fenbendazole is indicated (labeled) for the removal of the following parasites in swine: large roundworms (Ascaris suum), lungworms (Metastrongylus pair), nodular worms (Oesphagostomum dentatum, O. quadrispinolatum), small stomach worms (Hyostrongylus rubidus), whipworms (Trichuris suis), and kidney worms (Stephanurus dentatus; both mature and immature).Although not approved, fenbendazole has been used in cats, sheep, goats, pet birds, and llamas. |
|
Mode of action |
Fenbendazole is a benzimidazole antiparasitic drug that works at the sub-cellular level preventing cell division. Benzimidazoles bind to the β-tubulin, inhibiting the cell’s microtubule assembly responsible for intracellular transport and required for mitotic cellular division. In effect, it starves the parasite by causing intestinal cell disruption. |
|
Who Evaluation |
Evaluation year: 1999 |
InChI:InChI=1/C15H13N3O2S/c1-20-15(19)18-14-16-12-8-7-11(9-13(12)17-14)21-10-5-3-2-4-6-10/h2-9H,1H3,(H2,16,17,18,19)
43210-67-9 Relevant articles
Synthetic method of fenbendazole
-
Paragraph 0035-0052, (2021/08/14)
A synthetic method of fenbendazole belon...
Fenbendazole production process and production device
-
, (2021/01/24)
The invention discloses a fenbendazole p...
A preparation method of a dog or CAT
-
, (2019/03/29)
The invention discloses a dog or CAT a k...
Highly water-soluble prodrugs of anthelmintic benzimidazole carbamates: Synthesis, pharmacodynamics, and pharmacokinetics
Chassaing,Berger,Heckeroth,Ilg,Jaeger,Kern,Schmid,Uphoff
, p. 1111 - 1114 (2008/09/20)
Highly water-soluble prodrugs 1a-g of an...
43210-67-9 Process route
-
-
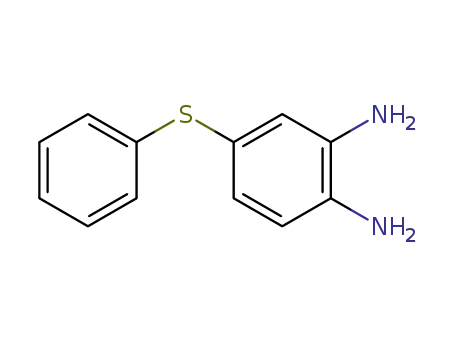
43156-48-5
1,2-diamino-4-phenylthiobenzene

-
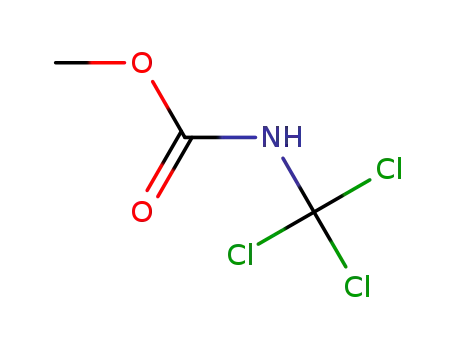
-
N-(trichloromethyl)carbamic acid methyl ester

-
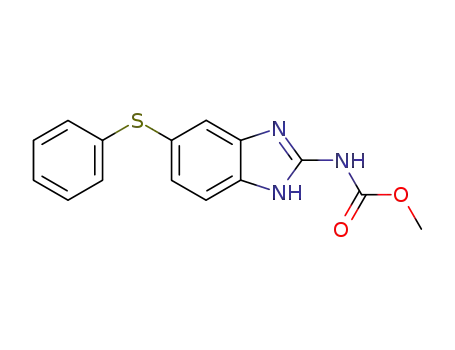
-
43210-67-9
Fenbendazole
| Conditions | Yield |
|---|---|
|
In
acetone;
at 40 - 60 ℃;
for 2.5h;
Temperature;
Time;
|
89.99% |
-

-
43156-48-5
1,2-diamino-4-phenylthiobenzene

-
-
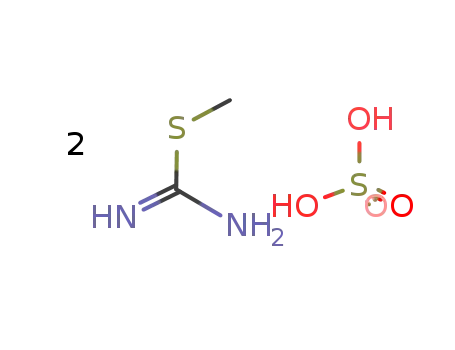
867-44-7,147895-43-0
S-Methylisothiourea sulfate

-
-
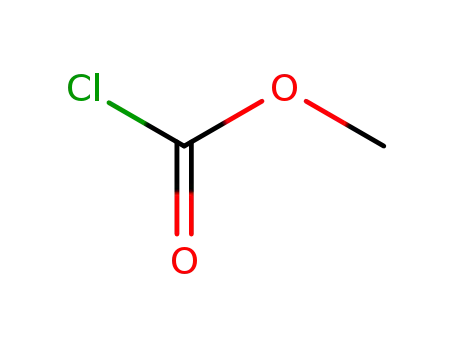
79-22-1
methyl chloroformate

-

-
43210-67-9
Fenbendazole
| Conditions | Yield |
|---|---|
|
S-Methylisothiourea sulfate; methyl chloroformate;
With
sodium hydroxide;
In
water;
at 3 - 6 ℃;
for 0.666667h;
1,2-diamino-4-phenylthiobenzene;
With
acetic acid;
In
ethanol; water;
at 95 ℃;
for 24h;
|
88% |
43210-67-9 Upstream products
-
6642-30-4
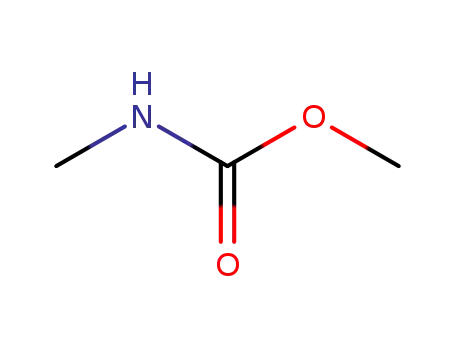
methyl N-methylcarbamate
-
43156-48-5

1,2-diamino-4-phenylthiobenzene
-
51686-78-3
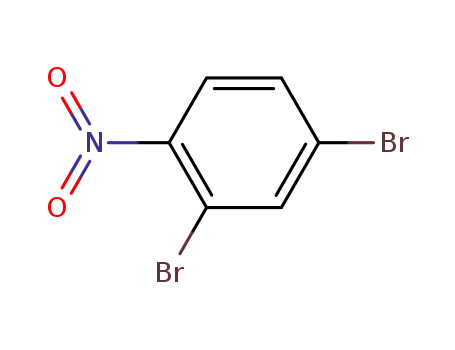
2,4-dibromonitrobenzene
-
106-37-6
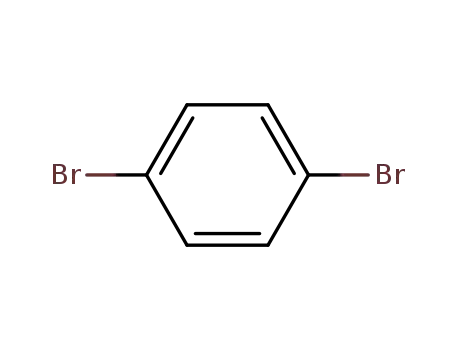
1.4-dibromobenzene
43210-67-9 Downstream products
-
53065-28-4
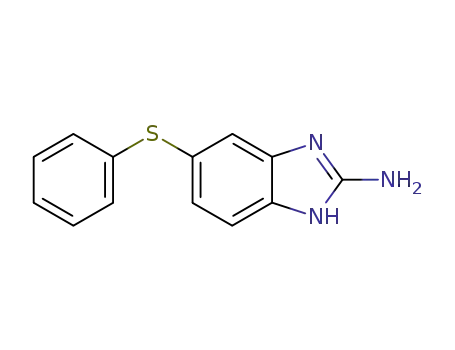
5-(phenylthio)-1H-benzo[d]imidazol-2-amine
-
58521-87-2
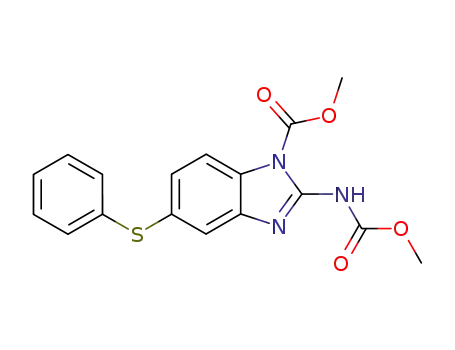
methyl 1-methoxycarbonyl-5-phenylthiobenzimidazole-2-carbamate
-
58522-04-6
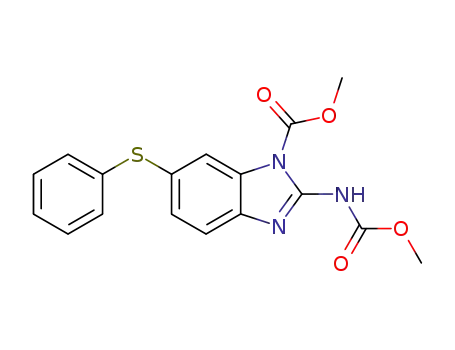
2-Methoxycarbonylamino-6-phenylsulfanyl-benzoimidazole-1-carboxylic acid methyl ester
-
104663-11-8
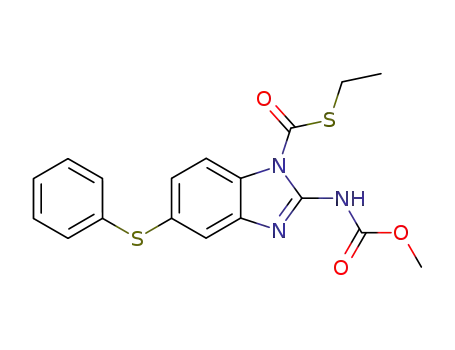
2-Methoxycarbonylamino-5-phenylsulfanyl-benzoimidazole-1-carbothioic acid S-ethyl ester