75-75-2
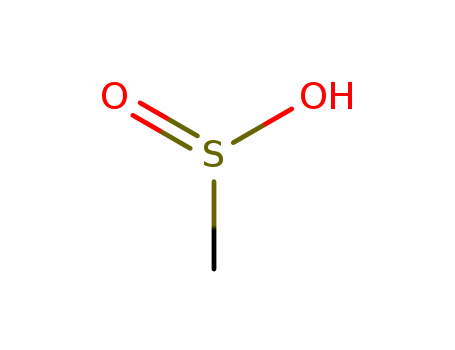
- Product Name:Methane sulfonic acid
- Molecular Formula:CH4O3S
- Purity:99%
- Molecular Weight:96.107
Reputable factory supply Methane sulfonic acid 75-75-2 in bulk at low price
- Molecular Formula:CH4O3S
- Molecular Weight:96.107
- Appearance/Colour:colourless or light yellow liquid
- Vapor Pressure:1 mm Hg ( 20 °C)
- Melting Point:19 °C
- Refractive Index:n20/D 1.429(lit.)
- Boiling Point:167 °C (10 torr)
- PKA:-2.6(at 25℃)
- Flash Point:>230 °F
- PSA:62.75000
- Density:1.511 g/cm3
- LogP:0.58480
Methanesulfonic acid(Cas 75-75-2) Usage
|
Preparation |
Methanesulfonic acid is produced predominantly by oxidizing methylthiol or dimethyl disulfide using nitric acid, hydrogen peroxide, chlorine or by employing electrochemical processes. |
|
Hazard |
Toxic by ingestion, skin irritant, corrosiveto tissue. |
|
Safety Profile |
Poison by ingestion and intraperitoneal routes. May be corrosive to skin, eyes, and mucous membranes. Explosive reaction with ethyl vinyl ether. Incompatible with hydrogen fluoride. When heated to decomposition it emits toxic fumes of SOx. See also SULFONATES. |
|
Purification Methods |
Dry the acid, either by azeotropic removal of water with *benzene or toluene, or by stirring 20g of P2O5 with 500mL of the acid at 100o for 0.5hours. Then distil it under vacuum and fractionally crystallise it by partial freezing. Sulfuric acid, if present, can be removed by prior addition of Ba(OH)2 to a dilute solution, filtering off the BaSO4 and concentrating under reduced pressure; and is sufficiently pure for most applications. [Beilstein 4 IV 10.] |
|
description |
Methanesulfonic acid (CH3SO3H, MSA) is a strong organic acid. The chemical oxidation of dimetyl sulfide in the atmosphere leads to the formation of MSA in large quantities. MSA undergoes biodegradation by forming CO2 and sulphate. It is considered a green acid as it is less toxic and corrosive in comparison to mineral acids.The aqueous MSA solution has been considered a model electrolyte for electrochemical processes.Methanesulfonic acid is an alkanesulfonic acid in which the alkyl group directly linked to the sulfo functionality is methyl. It has a role as an Escherichia coli metabolite. It is an alkanesulfonic acid and a one-carbon compound. It is a conjugate acid of a methanesulfonate. |
|
Chemical properties |
Methanesulfonic acid, the simplest alkanesulfonic acid, is a colorless or slightly brown oily liquid, appearing as solid at low temperatures. It has a melting temperature of 20 °C, the boiling point of 167 °C (13.33 kPa), 122 °C (0.133 kPa), ?the relative density of 1.4812 (18 ℃) and refractive index 1.4317 (16 ℃). It is soluble in water, alcohol and ether, insoluble in alkanes, benzene and toluene. It will not subject to decomposition in boiling water and hot alkaline solution. It also has strong corrosion effect against the metal iron, copper and lead. |
|
Definition |
ChEBI: An alkanesulfonic acid in which the alkyl group directly linked to the sulfo functionality is methyl. |
InChI:InChI=1/2CH4O3S.Fe/c2*1-5(2,3)4;/h2*1H3,(H,2,3,4);/q;;+2/p-2
75-75-2 Relevant articles
A high-yield approach to the sulfonation of methane to methanesulfonic acid initiated by H2O2 and a metal chloride
Mukhopadhyay, Sudip,Bell, Alexis T.
, p. 2990 - 2993 (2003)
Low temperatures and low pressures suffi...
Direct catalytic sulfonation of methane with SO2 to methanesulfonic acid (MSA) in the presence of molecular O2
Mukhopadhyay, Sudip,Bell, Alexis T.
, p. 1590 - 1591 (2003)
Methane is transformed selectively to me...
A novel method for the direct sulfonation of CH4 with SO 3 in the presence of KO2 and a promoter
Mukhopadhyay, Sudip,Bell, Alexis T.
, p. 754 - 757 (2003)
Direct sulfonation of methane with SO3 t...
75-75-2 Process route
-
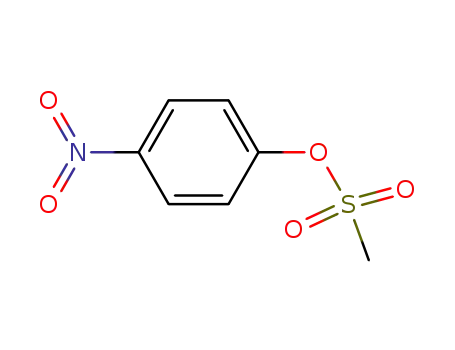
- 20455-07-6
p-nitrophenyl methanesulfonate

-
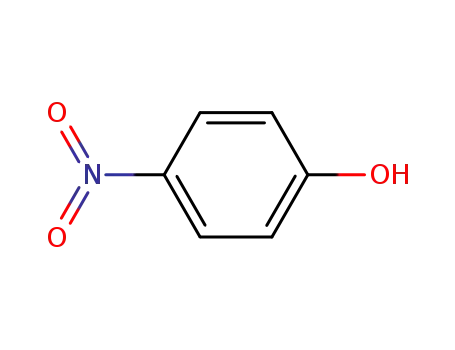
- 100-02-7,78813-13-5,89830-32-0
4-nitro-phenol

-
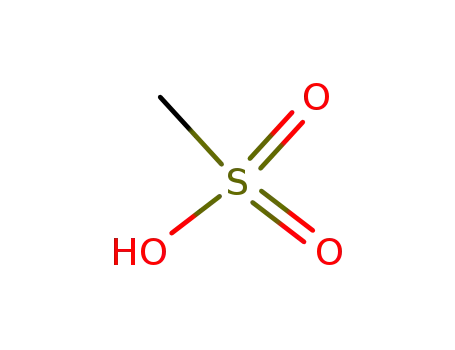
- 75-75-2,98527-29-8
methanesulfonic acid
| Conditions | Yield |
|---|---|
|
With water; hydroxide; In 1,4-dioxane; at 25 ℃; Rate constant;
|
-
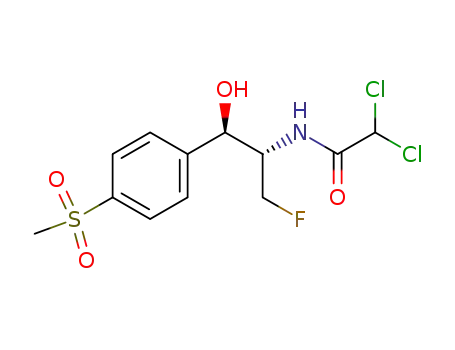
- 73231-34-2
Florfenicol

-

- 75-75-2,98527-29-8
methanesulfonic acid

-
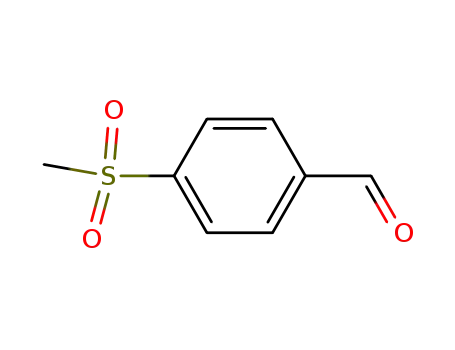
- 5398-77-6
para-methanesulfonylbenzaldehyde

-
-
C4H4Cl2FNO2

-
-
C12H13Cl3FNO4S

-
-
C11H11Cl3FNO2

-
-
C11H12Cl2FNO2

-
- 76639-93-5,105182-37-4,118015-48-8
(1R,2S)-2-amino-3-fluoro-1-<4-(methylsulphonyl)phenyl>-1-propanol

-
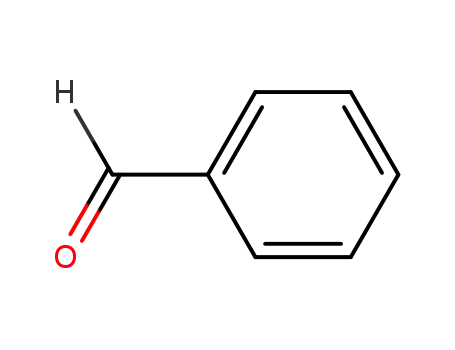
- 100-52-7
benzaldehyde

-
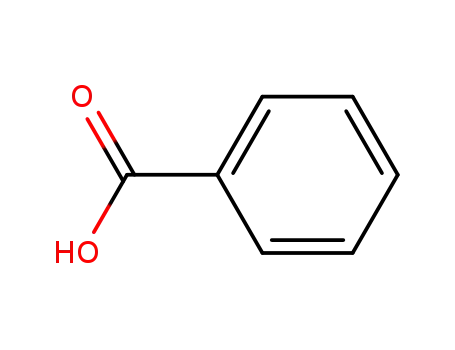
- 65-85-0,8013-63-6
benzoic acid
| Conditions | Yield |
|---|---|
|
With water; hypochloric acid; at 15 ℃; for 0.5h; pH=5.7; Reagent/catalyst; Kinetics;
|
75-75-2 Upstream products
-
123-91-1
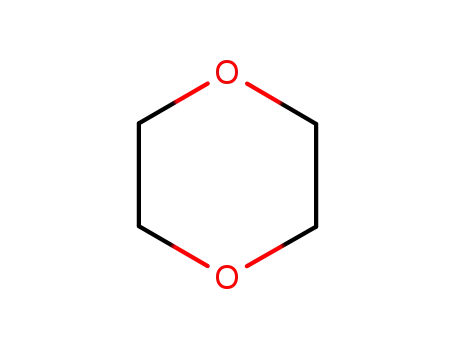
1,4-dioxane
-
93-59-4
Perbenzoic acid
-
67-66-3
chloroform
-
408309-55-7
bis-methanesulfonyl-bis-methylsulfanyl-methane
75-75-2 Downstream products
-
16156-54-0
sec-butyl methanesulfonate
-
2719-52-0
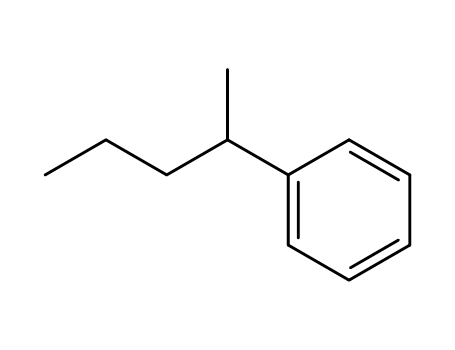
2-phenylpentane
-
1196-58-3
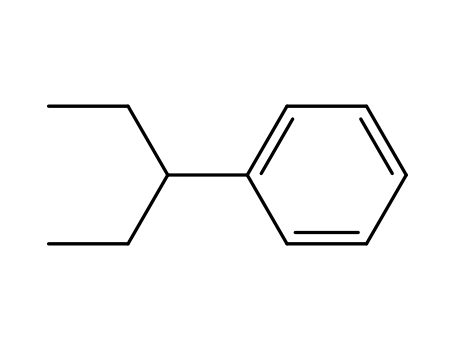
(1-ethylpropyl)benzene
-
68287-46-7
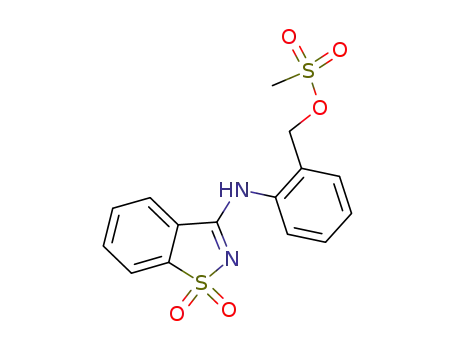
1-(1,1-dioxo-1H-1λ6-benzo[d]isothiazol-3-ylamino)-2-methanesulfonyloxymethyl-benzene