110-65-6
- Product Name:1,4-Butylene glycol
- Molecular Formula:C4H6O2
- Purity:99%
- Molecular Weight:86.0904
Reputable factory supply 1,4-Butylene glycol 110-65-6 in bulk at low price
- Molecular Formula:C4H6O2
- Molecular Weight:86.0904
- Appearance/Colour:white to light-brown solid or brownish-yellow aqueous solution
- Vapor Pressure:<0.1 mm Hg ( 55 °C)
- Melting Point:54 °C
- Refractive Index:1.4804
- Boiling Point:260.202 °C at 760 mmHg
- PKA:12.72±0.10(Predicted)
- Flash Point:135.558 °C
- PSA:40.46000
- Density:1.181 g/cm3
- LogP:-1.02560
2-Butyne-1,4-diol(Cas 110-65-6) Usage
|
Application |
Intermediate; corrosion inhibitor; electroplating brightener; defoliant ; polymerization accelerator; stabilizer for chlorinated hydrocarbons; cosolvent for paint and varnish removal. |
|
Definition |
ChEBI: A butynediol that is but-2-yne substituted by hydroxy groups at positions 1 and 4. |
|
General Description |
White to light-brown solid or brownish-yellow aqueous solution. Solid sinks and mixes with water. |
|
Air & Water Reactions |
Water soluble. |
|
Hazard |
Toxic by ingestion. May explode on con- tamination with mercury salts, strong acids, and alkaline earth hydroxides and halides at high tem- peratures. |
|
Health Hazard |
2-Butyne-1,4-diol exhibits moderate to hightoxicity in test animals. It is about 10 timesmore toxic than are the saturated C4 diols.The oral LD50 value in rats is 0.125 mL/kg.It causes irritation to the skin. |
|
Fire Hazard |
Noncombustible solid, flash point (open cup) 152°C (305.6°F). It is stable at room temperature. But the dry compound explodes in the presence of certain heavy metal salts, such as mercuric chloride. Heating with alkaline solution may result in an explosion. |
|
Flammability and Explosibility |
Nonflammable |
|
Safety Profile |
A poison by ingestion. A skin sensitizer upon long or repeated contact. Moderately explosive. When heated to decomposition it emits acrid smoke and fumes and may explode. Explosive reaction with traces of alkaltes, alkali earth hydroxides, halide salts, strong acids, mercury salts + strong acids. See also ACETYLENE COMTOUNDS. |
|
Synthesis |
2-butyne-1,4-diol is carried out by reaction under pressure of acetylene and an aqueous solution of formaldehyde, catalyzed by copper acetylide. |
|
Purification Methods |
Crystallise the diol from EtOAc. [Beilstein 1 IV 2687.] |
InChI:InChI=1/C4H6O2/c1-2-3-4(5)6/h4-6H,1H3
110-65-6 Relevant articles
Walter Reppe Revival – Identification and Genesis of Copper Acetylides Cu2C2 as Active Species in Ethynylation Reactions
Bruhm, Tobias,Abram, Andrea,H?usler, Johannes,Thomys, Oliver,K?hler, Klaus
, p. 16834 - 16839 (2021)
More than six decades after proposing co...
Tetrabutylammonium tribromide (TBATB) - MeOH: An efficient chemoselective reagent for the cleavage of tert-butyldimethylsilyl (TBDMS) ethers
Gopinath, Rangam,Patel, Bhisma K.
, p. 4177 - 4180 (2000)
(equation presented) R = H, alkyl or ary...
Syntheses of 7-Substituted Anthra[2,3- b]thiophene Derivatives and Naphtho[2,3- b:6,7- b']dithiophene
Al-Jumaili, Mustafa A.,Woodward, Simon
, p. 11437 - 11445 (2018)
7-R-Anthra[2,3-b]thiophene derivatives (...
110-65-6 Process route
-
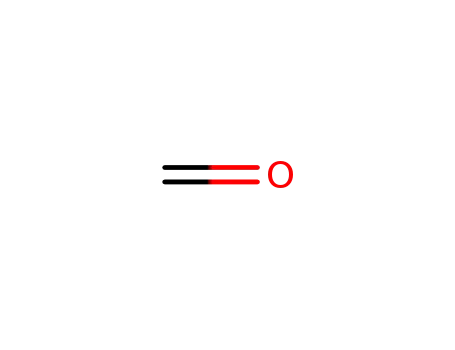
- 50-00-0,30525-89-4,61233-19-0
formaldehyd

-
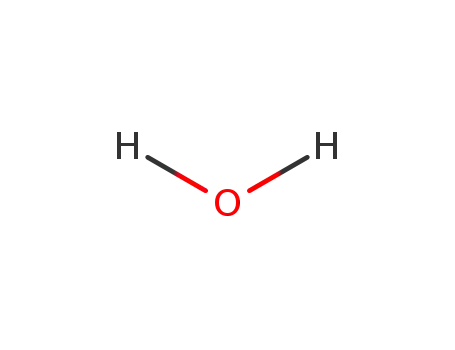
- 7732-18-5
water

-
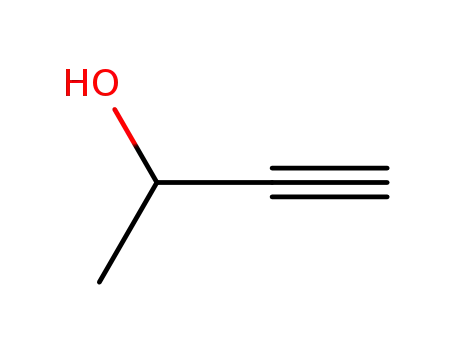
- 2028-63-9
but-3-yn-2-ol

-
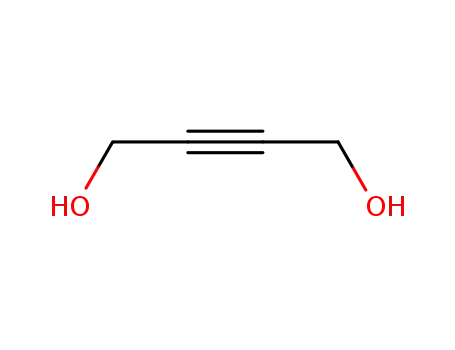
- 110-65-6
1,4-dihydroxybut-2-yne

-
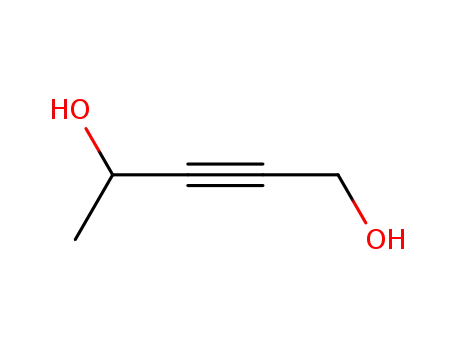
- 927-57-1
pent-2-yne-1,4-diol

-
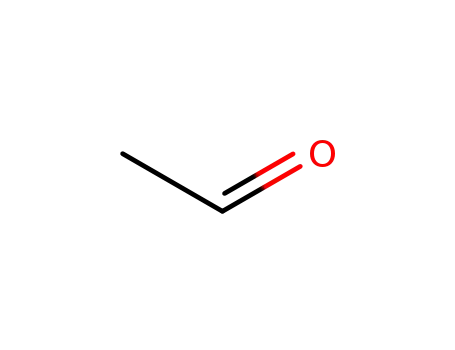
- 75-07-0,9002-91-9
acetaldehyde

-
- 74-86-2,25067-58-7
acetylene
| Conditions | Yield |
|---|---|
|
|
-
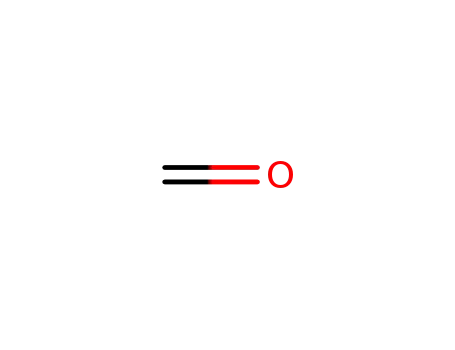
- 50-00-0,30525-89-4,61233-19-0
formaldehyd

-
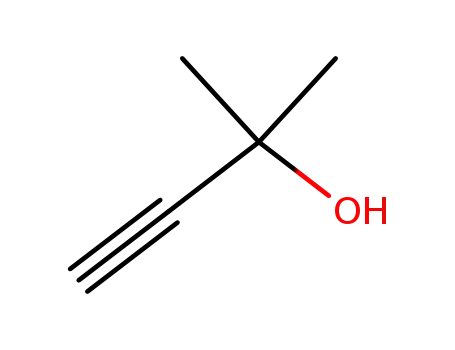
- 115-19-5
2-methyl-but-3-yn-2-ol

-

- 110-65-6
1,4-dihydroxybut-2-yne

-
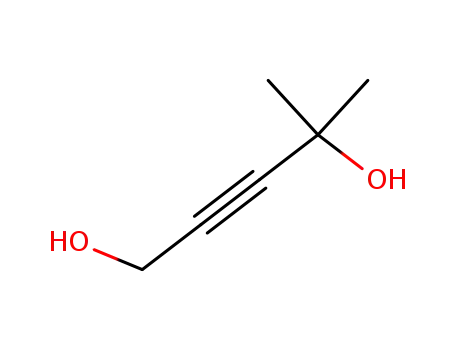
- 10605-66-0
4-methyl-2-pentyn-1,4-diol

-
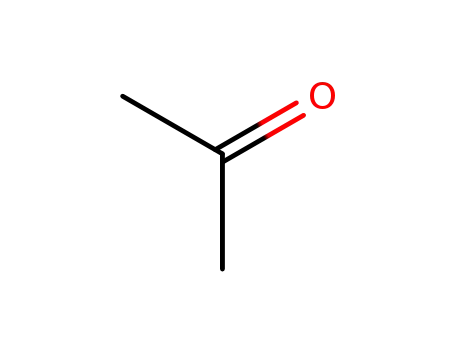
- 67-64-1
acetone
| Conditions | Yield |
|---|---|
|
|
110-65-6 Upstream products
-
110-88-3
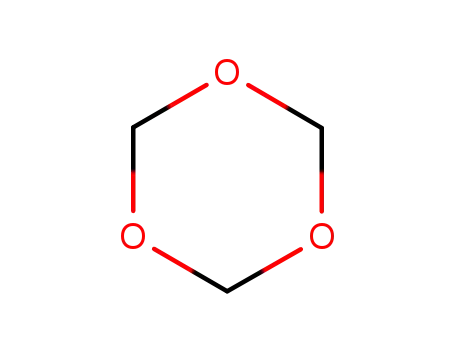
1,3,5-Trioxan
-
4301-15-9
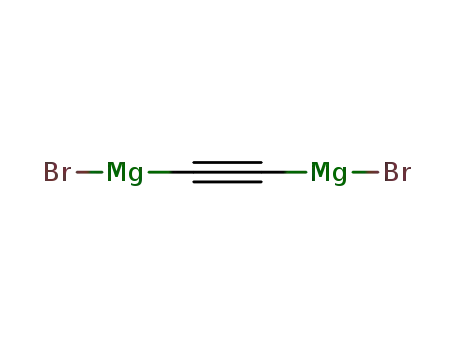
ethynyldimagnesium dibromide
-
3234-02-4
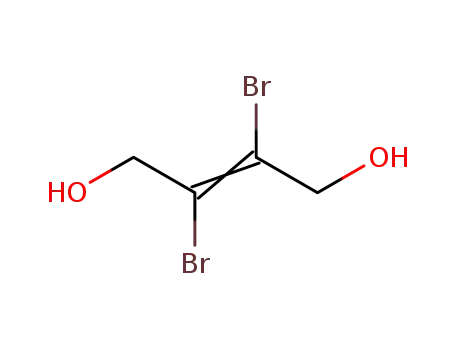
2,3-dibromo-but-2-ene-1,4-diol
-
50-00-0
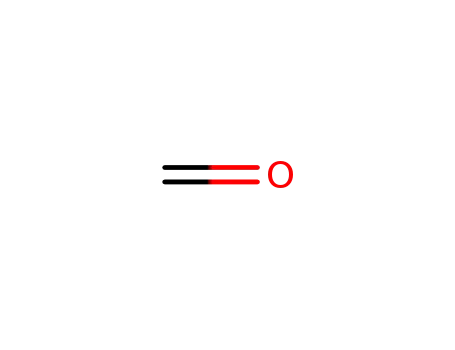
formaldehyd
110-65-6 Downstream products
-
16356-02-8
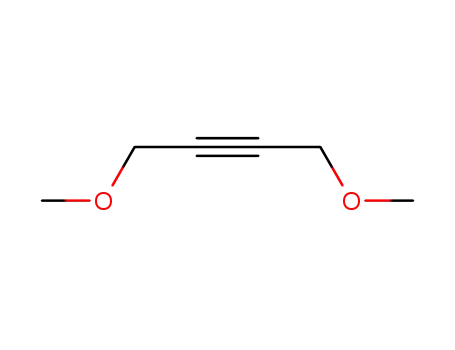
1,4-dimethoxy-2-butyne
-
2798-71-2
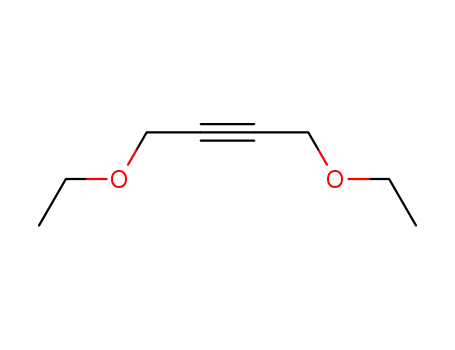
Butindiol-diethylether
-
18857-03-9
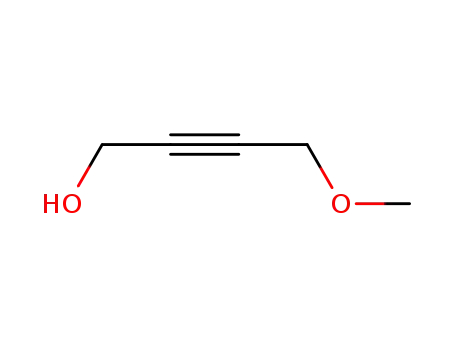
4-methoxy-but-2-yn-1-ol
-
210345-25-8
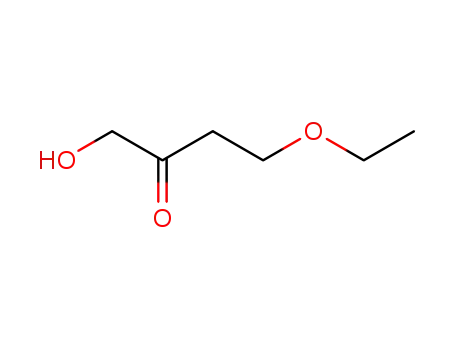
4-ethoxy-1-hydroxy-butan-2-one